[Literature Review] CRISPR KO Screening Aids in Developing Potential Antidotes for Cobra Venom Bites
CRISPR gene screening
Cobra venom envenomation is a serious medical emergency as its toxins can rapidly affect the nervous system, blood system, and tissues. Finding effective antidotes is crucial in treating cobra venom bites.
In recent years, researchers have made some progress in exploring potential antidotes. For example, certain natural plant extracts, antivenom serums, and synthetic drugs have been evaluated as potential antidotes.
These antidotes help mitigate harm by neutralizing the toxins, blocking their action, or promoting toxin elimination. Although there is no completely ideal antidote currently, ongoing research by scientists offers hope for future treatment options.
In July this year, researchers from the University of Sydney, Liverpool School of Tropical Medicine, University of Costa Rica, and other institutions jointly published a research paper titled "Molecular dissection of cobra venom highlights heparinoids as an antidote for spitting cobra envenoming" in the journal Science Translational Medicine (Q1, Top, IF: 17.1), a subsidiary of Science.
Snake bites affect approximately 1.8 million people annually. The current standard treatment involves antibody-based antivenoms, which can be difficult to obtain and are often ineffective against local tissue damage, a major cause of morbidity.
Using whole-genome CRISPR gene screening , researchers identified several key genes affecting cellular response to cobra venom, including EXT1, B4GALT7, EXT2, EXTL3, XYLT2, NDST1, and SLC35B2.
Independent verification by the researchers found that heparinoid drugs might act as inhibitors , indicating that these drugs could be potential therapies for spitting cobra envenomation.

Original Article Link: https://www.science.org/doi/10.1126/scitranslmed.adk4802
According to surveys, snake bites result in 138,000 deaths and leave 400,000 people with long-term disabilities annually, particularly affecting young people and children in sub-Saharan Africa and South Asia.
As one of the deadliest neglected tropical diseases, snake bite envenomation imposes a severe burden primarily on poor rural communities, with the disease burden in West Africa and Southeast Asia amounting to billions of dollars.
In response to this crisis, the World Health Organization has elevated snake bite envenoming to a “Category A Neglected Tropical Disease” priority and set a goal to halve the global burden of snake bites by 2030.
Although existing antivenoms can save lives, they are expensive and ineffective against local tissue damage. To address this issue, researchers have employed functional genomics to define venom-targeted gene interactions that alter cellular toxicity caused by spitting cobra venom, using this information to develop a locally acting venom antidote.
I. Whole-Genome CRISPR Knockout Screening of Cobra Venom Cytotoxicity
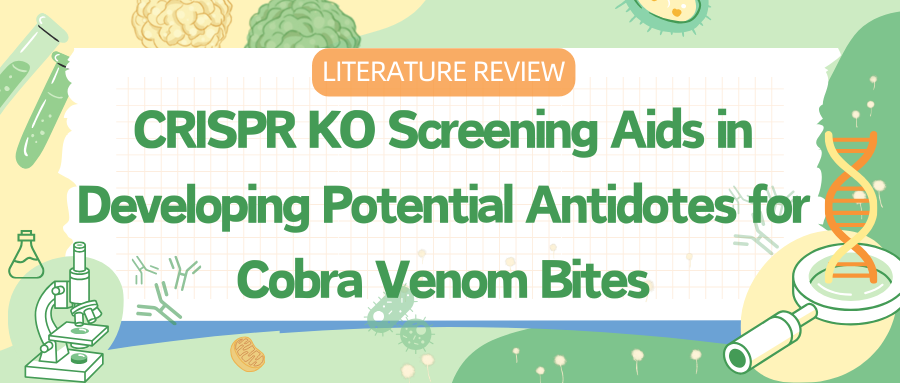
Researchers first tested the cytotoxicity of venoms from the Red Spitting Cobra (Naja pallida, Tanzania) and the Black-necked Spitting Cobra (Naja nigricollis, Nigeria) on the human haploid cell line HAP1.
Additional pharmacological inhibition with caspase-3 inhibitor Ac-DEVD-Cho or pan-caspase inhibitor Z-VAD-FMK did not inhibit the cytotoxicity of the snake venoms. However, the necroptosis inhibitor Necrosulfonamide limited some cell death, suggesting that the cytotoxicity of cobra venoms may partially induce necroptotic cell death.
To guide the development of therapeutic drugs, researchers used whole-genome CRISPR knockout screening to study the molecular mechanisms of snake venom-induced cell death. By transducing HAP1 cells with the TKOv3 library, which targets most human protein-coding genes, and treating the cells with venoms from Naja pallida and Naja nigricollis, they identified genes sensitive and resistant to the venoms.
The cytotoxic mechanisms of venoms from Naja pallida and Naja nigricollis mainly involve specific genes and biosynthetic pathways, especially the biosynthesis of heparan sulfate/heparin. Key biosynthetic pathways include the synthesis of heparan sulfate, chondroitin sulfate, and dermatan sulfate.
Results show that targeting most components of the heparan sulfate biosynthetic pathway alone is sufficient to block venom activity.
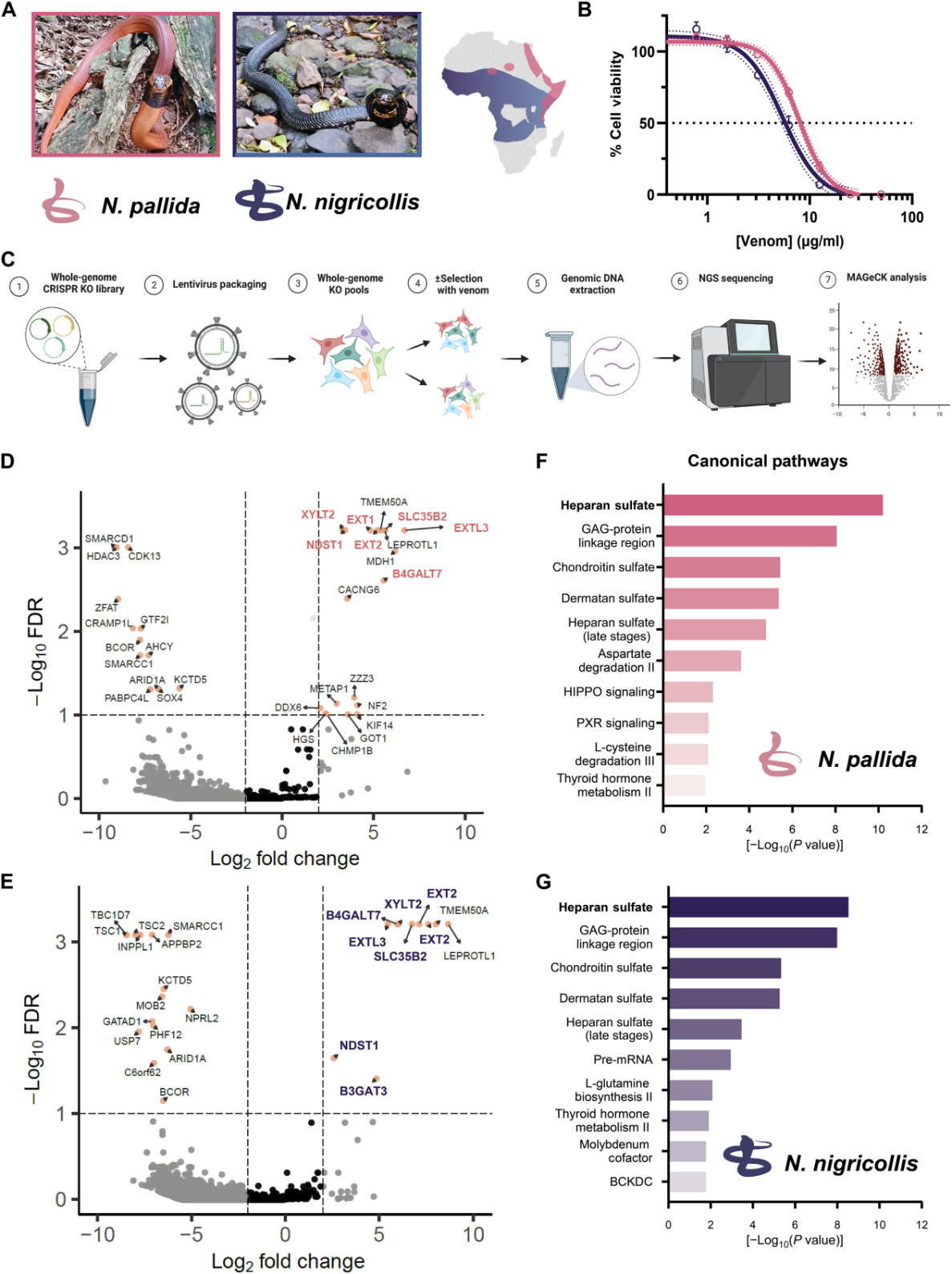
Figure 1: Whole-Genome CRISPR-Cas9 KO Screening Identifies Genes Required for the Cytotoxicity of African Spitting Cobra Venom In Vitro
II. Heparan Sulfate Biosynthesis is Essential for Venom Cytotoxicity
To further validate the ability of components of the heparan sulfate/heparin biosynthetic pathway to block venom activity, researchers generated a series of gene knockout cell pools targeting different genes within the heparan sulfate/heparin biosynthetic pathway and tested the response of these cells to venom.
The results confirmed the role of heparan sulfate in the cytotoxicity of cobra venom. Subsequently, researchers treated these gene knockout cells with venom from cobras (N. nigricollis, Tanzania) from different regions. The results again indicated that components of the heparan sulfate/heparin biosynthetic pathway are essential for venom cytotoxicity.
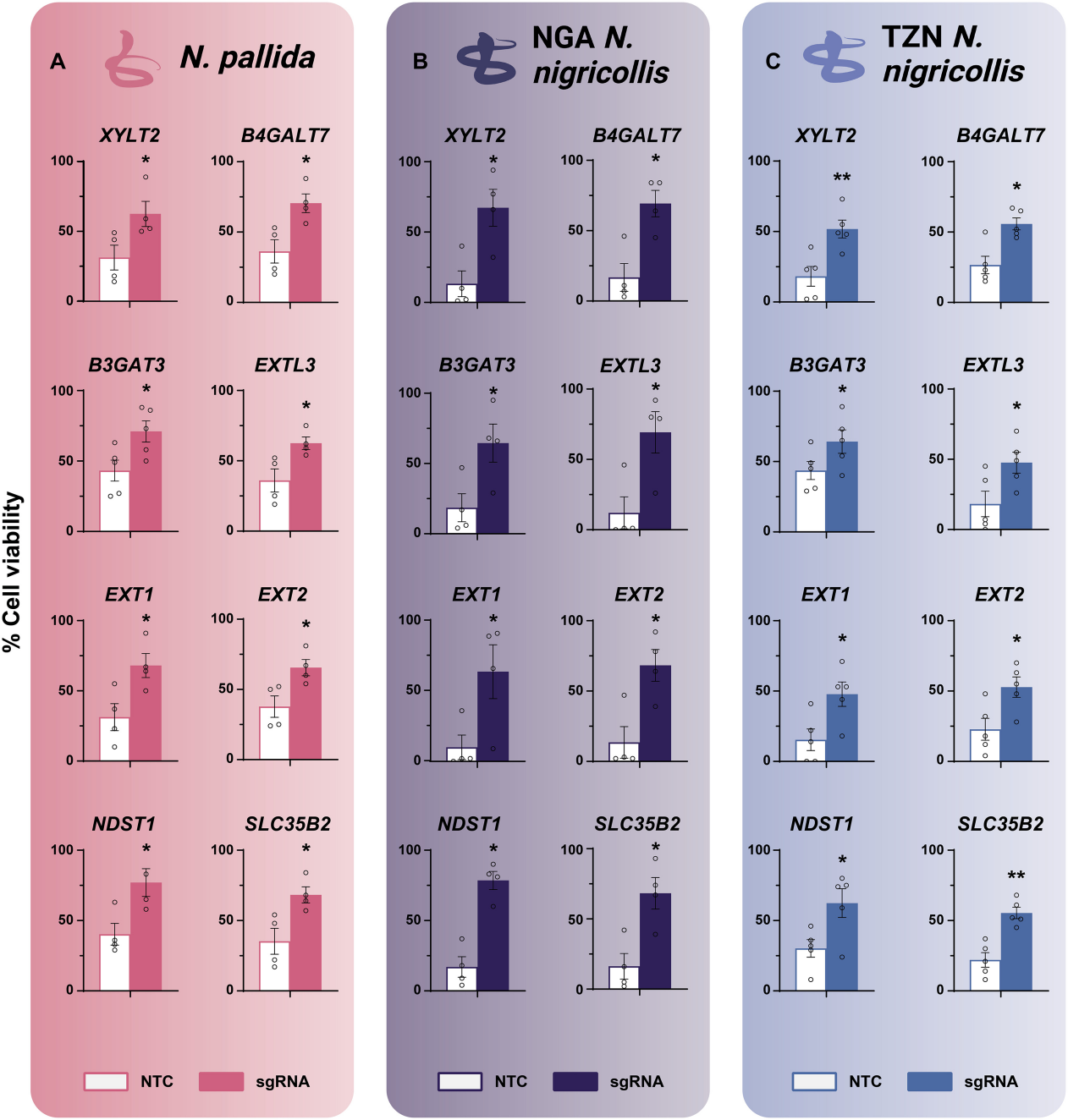
Figure 2: Heparan Sulfate Biosynthesis is Essential for the Cytotoxicity of Spitting Cobra Venom
Heparin and heparan sulfate share a sugar backbone synthesized through a common pathway. Heparan sulfate is a ubiquitous component of the extracellular matrix, while heparin is primarily produced by tissue mast cells.
Heparin is a highly sulfated polyanionic polysaccharide used clinically for its potent anticoagulant activity. To verify this, researchers also used heparin analogs, such as heparin, tinzaparin, or dalteparin.
They found that excess heparin or low molecular weight heparin could effectively block the cytotoxicity of the venom, and these drugs also had inhibitory effects on different types of venoms.
Additionally, when cells were treated with venom from N. nigricollis, the results confirmed that heparin or tinzaparin treatment could also block the cytotoxicity induced by venoms from N. pallida and N. nigricollis (Tanzania).
Figure 3: Heparin and LMW Heparin Block the Effects of Naja Venom In Vitro
III. Heparin-like Drugs Prevent Venom Interaction with Cell Surfaces
Heparan sulfate and its analogs can act as venom antidotes by serving as "decoy" receptors, blocking the interaction between cobra venom and host cells.
Further validation showed that labeled cobra venom strongly binds to untreated cells, whereas the addition of heparan sulfate or tinzaparin significantly reduced this binding, blocking the cytotoxic effects of the venom. This conclusion was confirmed with venoms from different geographical variants of cobras.
The results indicate that free heparin can inhibit venom-target interactions, which is sufficient to block cytotoxicity.
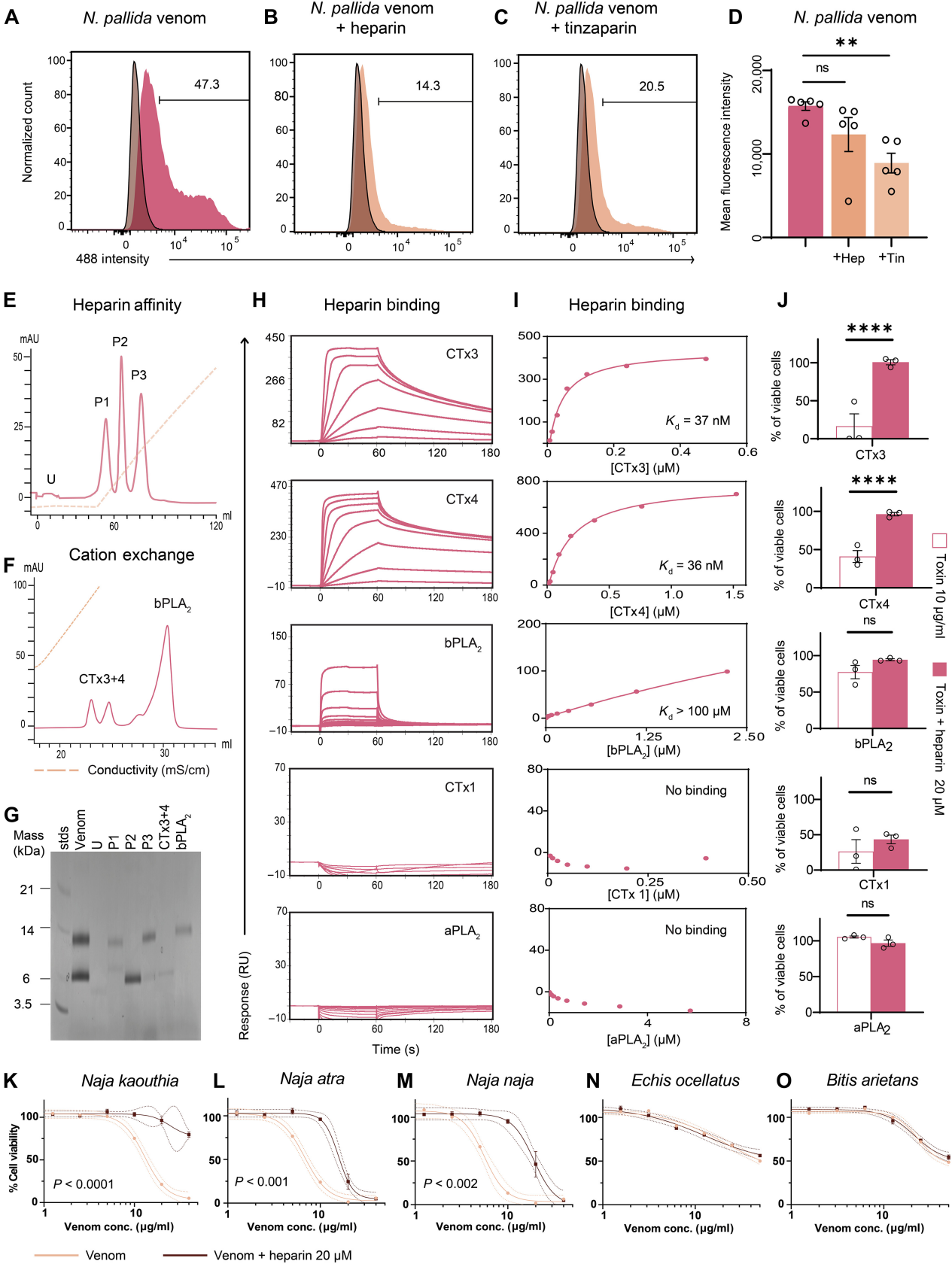
Figure 4: Heparin Binds 3FTxs and Prevents Their Cytotoxicity
IV. Heparin Interacts with Three-Finger Cytotoxins, Blocking Venom-Host Interactions
Cobra venom primarily consists of phospholipase A2 (PLA2) and three-finger cytotoxins (3FTxs). Researchers used heparin affinity chromatography to isolate different components of cobra venom and identified the major proteins via liquid chromatography-mass spectrometry. The results showed that 3FTxs CTx3 and CTx4 have high affinity for heparin, while PLA2 has weaker binding affinity.
Further experiments demonstrated that heparin effectively inhibits the cytotoxicity of 3FTxs CTx3 and CTx4, but has little effect on other toxins like CTx1. Heparin and N-acetylheparin can block the cytotoxicity of various cobra venoms but are ineffective against the venoms of other snakes such as the saw-scaled viper and puff adder. This suggests that heparin and its low-molecular-weight derivatives could be potential antidotes for cobra venom.
V. Heparin-like Drugs Prevent Cobra Venom-Induced Skin Damage
Researchers found that heparin and heparin-like drugs can protect human epidermal keratinocytes from cobra venom-induced cytotoxicity. While snake venom induces cell death, heparin improves cell survival rates.
In vivo tests showed that heparin-like drugs significantly reduced cobra venom-induced skin necrosis, with tinzaparin being the most effective, reducing lesion areas by an average of 94%.
Further studies indicated that different doses of tinzaparin effectively reduced the size of necrotic skin lesions and mitigated damage to skin layers. The research suggests that heparin-like drugs can prevent severe local skin necrosis caused by cobra venom by blocking certain components of the venom.
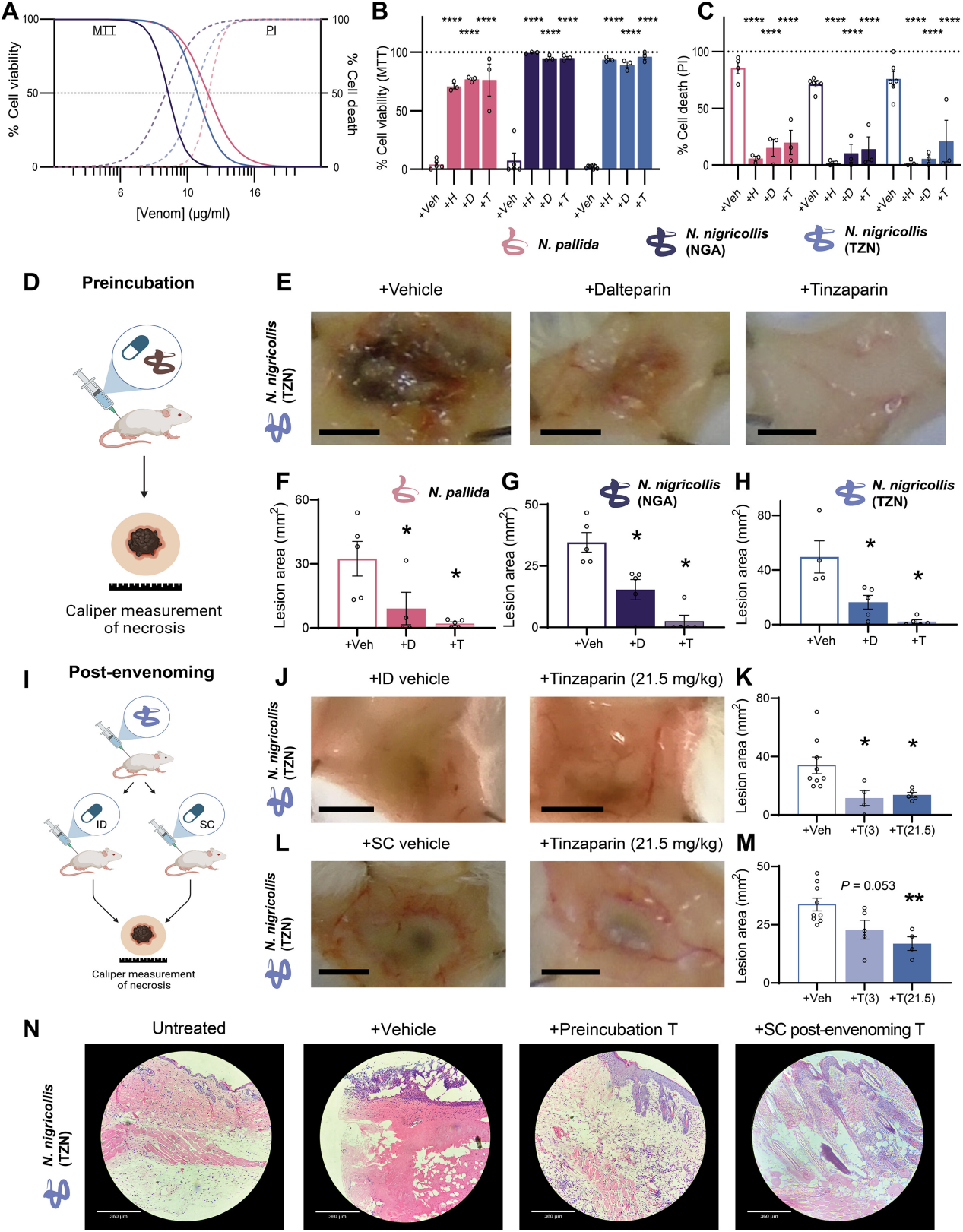
Figure 5: Heparin-like Drugs Inhibit Snake Venom-Induced Skin Necrosis In Vivo
In summary, researchers used CRISPR library screening technology to identify several genes and pathways crucial for snake venom cytotoxicity, including the biosynthesis of heparan sulfate/heparin.
The study revealed that heparin-like drugs can prevent skin necrosis associated with cobra venom, providing a potential therapeutic approach for spitting cobra venom.
See how you can utilize CRISPR library screening technology in your research for target genes and pathways. Check out EDITGENE’s complete solution for CRISPR library screening , CRISPR KO/CRISPRa/CRISPRi , and the most extensive collection of library plasmids/viruses, which is available within weeks.
Recent blogs :
3. [Literature Review] Multiplex Detection Strategy of Biosensors Based on CRISPR-Cas System
Follow us on social media
Contact us
+ 833-226-3234 (USA Toll-free)
+1-224-345-1927 (USA)
info@editxor.com














Comment (4)