 iPSC Gene Editing
iPSC Gene Editing
Induced pluripotent stem cells (iPSCs), with their capacity for unlimited self-renewal and differentiation into diverse cell types, have become a powerful tool for studying human development, modeling diseases, and enabling drug discovery. By introducing precise genetic modifications in iPSCs, such as knockouts, knock-ins, point mutations, or gene corrections, researchers can replicate disease states at the genetic level, investigate pathogenic mechanisms, and build a strong foundation for personalized therapies and regenerative medicine.
At EDITGENE, we leverage our proprietary CRISPR/Cas9, TALEN, and Prime Editing platforms to deliver iPSC gene-editing services with high efficiency and minimal off-target effects. Through rigorous single-cell cloning and multi-level validation, including sequencing and differentiation potential assays, we ensure the accuracy and long-term stability of our edited iPSC lines.Service Details
| Services | Gene-edited iPS cells: knockout, knock-in, point mutation, overexpression, Knockdown |
|---|---|
| Deliverables | Monoclonal cell ≥ 1 strain (2 tubes of cells/strain, 1x106/tube) |
| Turnaround/Price |
As fast as 12 weeks,as low as $6800 |
Service Types
EDI-Service Advantages
Extensive Experience in Stem Cell Cultivation
Advanced Gene Editing Platform
Designing target gene editing iPS cell models based on experimental needs.
Case Study
Establishing a Point Mutation Cell Model in iPSCs Using the Prime Editing Platform
| Cell Name | cell pool editing efficiency | Sequencing peak profile |
|---|---|---|
| WT cell | / |
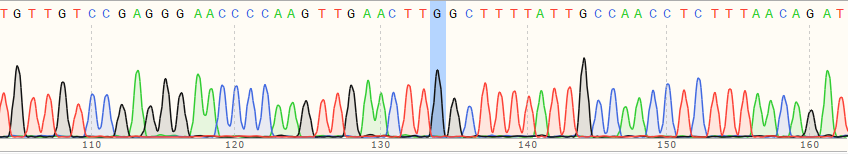 |
| A Polyclonal Cells | 10% |
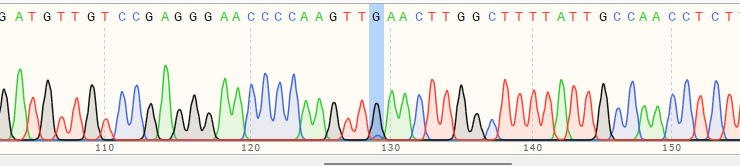 |
| B Polyclonal Cells | 55% |
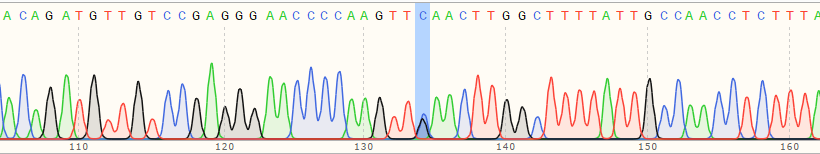 |
| C Polyclonal Cells | 17% |
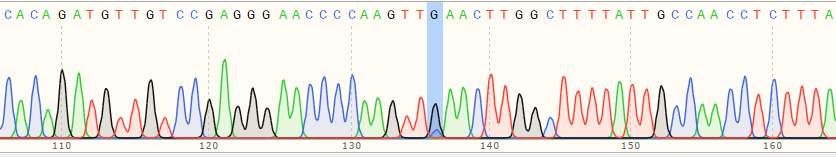 |
| Monoclonal Cell | 100% |
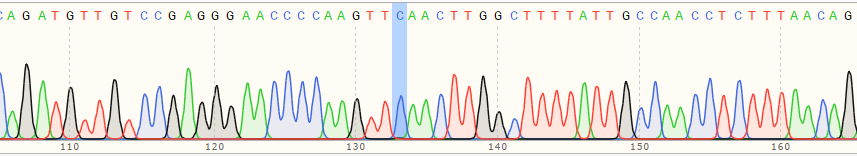 |
Generation of a Knockout iPSC Model Using the CRISPR/CAS9 Platform
| Cell Name | cell pool editing efficiency | Sequencing peak profile |
|---|---|---|
| WT cell | / |
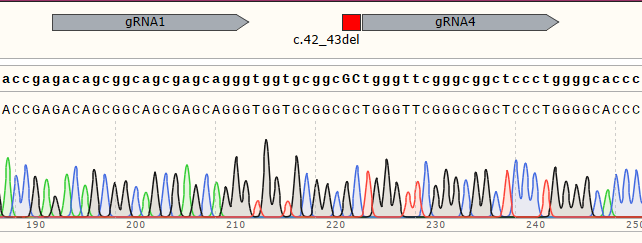 |
| Polyclonal Cells | 98% |
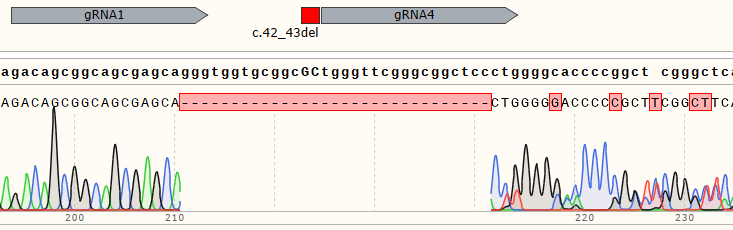 |
| Monoclonal Cell | 100% |
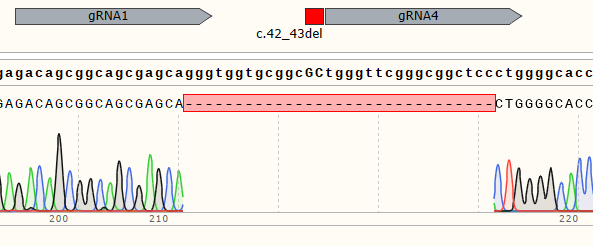 |
Advantage and Characteristic

Optimazied Strategy

Optimazied Strategy

Optimazied Strategy

Optimazied Strategy
Reference Materials
Four key factors, Oct4, Sox2, Klf4, and c-Myc, can induce cell reprogramming into iPSCs
This article discusses the technology of converting mature fibroblasts into pluripotent stem cells through specific transcription factors. The study utilized four key transcription factors—Oct4, Sox2, Klf4, and c-Myc—introduced into mouse embryonic and adult fibroblasts. Through the action of these factors, the cells gradually acquired pluripotent characteristics, exhibiting morphology and gene expression patterns similar to embryonic stem cells. The study found that the reprogrammed iPSCs could not only be maintained long-term in vitro but also differentiate into various cell types, including neural cells and cardiomyocytes, indicating that these iPSCs have favorable biological characteristics and potential applications. This study validates the effectiveness of these four transcription factors and provides new tools for future stem cell research, especially in regenerative medicine and disease treatment.
iPSC-derived lung and lung cancer organoid model for testing drug delivery efficacy
Lung cancer remains the leading cause of cancer-related mortality worldwide. Despite advancements in targeted therapies, drug resistance and systemic toxicity are persistent issues. This study investigates the feasibility of using patient-specific lung cancer and normal lung tissue organoid models, as well as autologous induced pluripotent stem cell (iPSC)-derived mesenchymal stromal cell (MSC)-isolated extracellular vesicles (EVs) in personalized medicine. Healthy fibroblasts were reprogrammed into iPSCs, which differentiated into branching lung organoids (BLO) and patient-matched lung cancer organoids (LCO). EVs were isolated from iPSC-MSCs and loaded with 0.07 µg/mL anticancer drugs(CDDP), applied to both organoid models, and cytotoxicity was recorded through LDH and CCK8 assays. The results showed that fibroblast-derived iPSCs exhibited a normal karyotype and pluripotency, while iPSC-derived BLOs and LCOs expressed lung markers. CDDP-loaded iPSC-MSC-derived EVs caused no cytotoxicity in either organoid model, whereas 20 µg/mL CDDP was cytotoxic to LCOs. This study presents an initial validation method for using autologous or allogeneic iPSC-MSC EVs as a testing platform for lung cancer drug delivery.
iPSC-derived macrophages as effective models for studying viral biology
This study assesses whether human iPSC-derived macrophages serve as effective models for studying viral biology. The findings show that these iPSC-derived macrophages support the replication of HIV-1, dengue, and influenza viruses, with replication dynamics and phenotypes comparable to traditional blood monocyte-derived macrophages. Flow cytometry, RNA sequencing, and chromatin accessibility analyses showed that iPSC-derived macrophages closely resemble human blood monocyte-derived macrophages in surface markers and gene expression characteristics. Additionally, iPSC lines generated from chimpanzee fibroblasts exhibited different susceptibilities to dengue virus, providing a valuable resource for studying viral host tropism. The study also found that both blood-derived and iPSC-derived macrophages could restrict viral replication in the late stage of the influenza virus lifecycle. Overall, iPSC-derived macrophages have proven to be a suitable alternative to blood monocyte-derived macrophages for studying viral biology.
Selected Customer Resources
Deep whole-genome analysis of 494 hepatocellular carcinomas
Abstract:
To date, more than half of global hepatocellular carcinoma (HCC) cases occur in China, yet comprehensive whole-genome analyses focusing on HBV-related HCC within the Chinese population remain scarce. To address this challenge, researchers initiated the China Liver Cancer Atlas (CLCA) project, aiming to conduct large-scale whole-genome sequencing to unravel the unique pathogenic mechanisms and evolutionary trajectories of HCC in China.
The researchers performed deep whole-genome sequencing on 494 HCC tumor samples, with an average depth of 120×, alongside matched blood controls, providing a detailed genomic landscape of HBV-associated HCC. Beyond confirming well-known coding driver genes such as TP53 and CTNNB1, the study identified six novel coding drivers—including FGA—and 31 non-coding driver genes.
Additionally, the research uncovered five new mutational signatures, including SBS_H8, and characterized the presence of extrachromosomal circular DNA (ecDNA) formed via HBV integration, which contributes to oncogene amplification and overexpression. Functional validation experiments demonstrated that mutations in genes such as FGA, PPP1R12B, and KCNJ12 significantly enhance HCC cell proliferation, migration, and invasion.
These findings not only deepen our insights into the genomics of HCC, but also open up new potential targets for diagnosis and therapy. View details>>
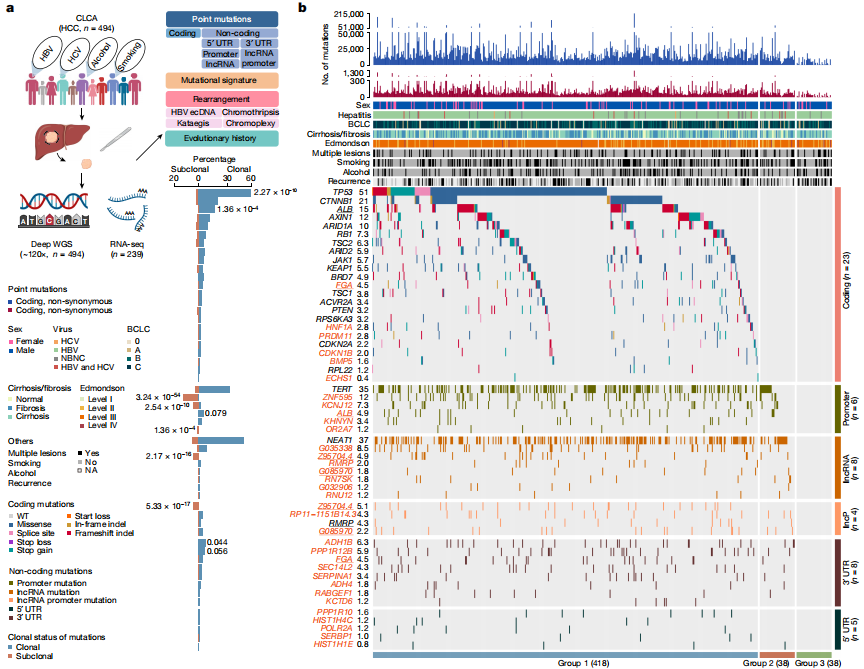
Candidate driver landscape
Targeted Macrophage CRISPR-Cas13 mRNA Editing in Immunotherapy for Tendon Injury
Abstract:
During the acute inflammatory phase of tendon injury, excessive activation of macrophages leads to the overexpression of SPP1, which encodes osteopontin (OPN), thereby impairing tissue regeneration. The CRISPR-Cas13 system holds great promise for tissue repair due to its unique RNA editing and rapid degradation capabilities; however, its application has been limited by the lack of efficient delivery methods.
To address this, the researchers systematically screened various cationic polymers targeting macrophages and developed a nanocluster carrier capable of efficiently delivering Cas13 ribonucleoprotein complexes (Cas13 RNPs) into macrophages. Utilizing a reactive oxygen species (ROS)-responsive release mechanism, this system specifically suppresses the overexpression of SPP1 in macrophages within the acute inflammatory microenvironment of tendon injury.
Experimental results demonstrated that this targeted delivery strategy significantly reduced the population of SPP1-overexpressing macrophages induced by injury, inhibited fibroblast activation, and alleviated peritendinous adhesion formation. Furthermore, the study elucidated that SPP1 promotes fibroblast activation and migration through the CD44/AKT signaling pathway, and that inhibiting this pathway effectively mitigates adhesion formation following tendon injury. View details>>
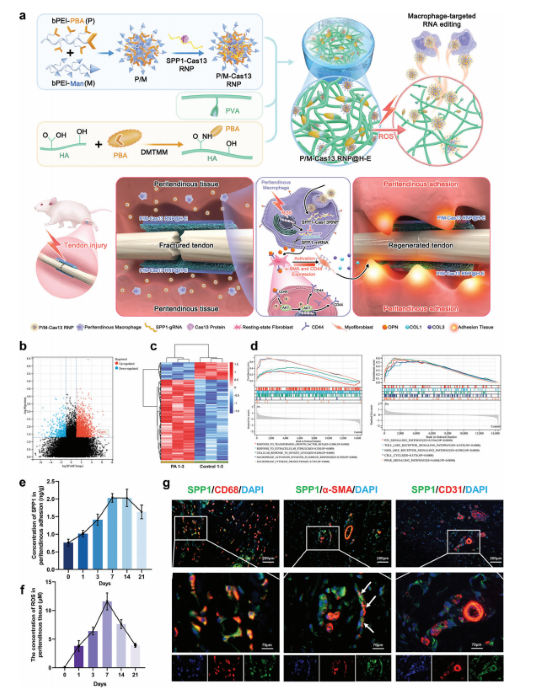
Schematic diagram illustrating immune microenvironment-activated mRNA editing strategies of macrophages for PA therapy
Electrical stimulation of piezoelectric BaTiO3 coated Ti6Al4V scaffolds promotes anti-inflammatory polarization of macrophage and bone repair via MAPK/JNK inhibition and OXPHOS activation
Abstract:
Spinal cord injury (SCI) is a severe disabling condition that causes permanent loss of sensory, autonomic, and motor functions. While stem cell therapies, particularly mesenchymal stem cells (MSCs), show great promise for SCI treatment, their limited regenerative capacity restricts their application in tissue repair. The researchers observed that extracellular vesicles derived from antler bud progenitor cells (EVsABPC) may carry bioactive signals that promote tissue regeneration. Accordingly, they isolated and engineered EVs from ABPCs for SCI therapeutic investigation.
The study found that EVsABPC significantly enhanced neural stem cell (NSC) proliferation, promoted axonal growth, reduced neuronal apoptosis, and modulated inflammation by shifting macrophage polarization from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype. Moreover, engineered EVsABPC modified with cell-penetrating peptides demonstrated improved targeting to the SCI lesion site, markedly enhancing neural regeneration and functional motor recovery. These findings highlight EVsABPC as a promising candidate for SCI therapy. View details>>
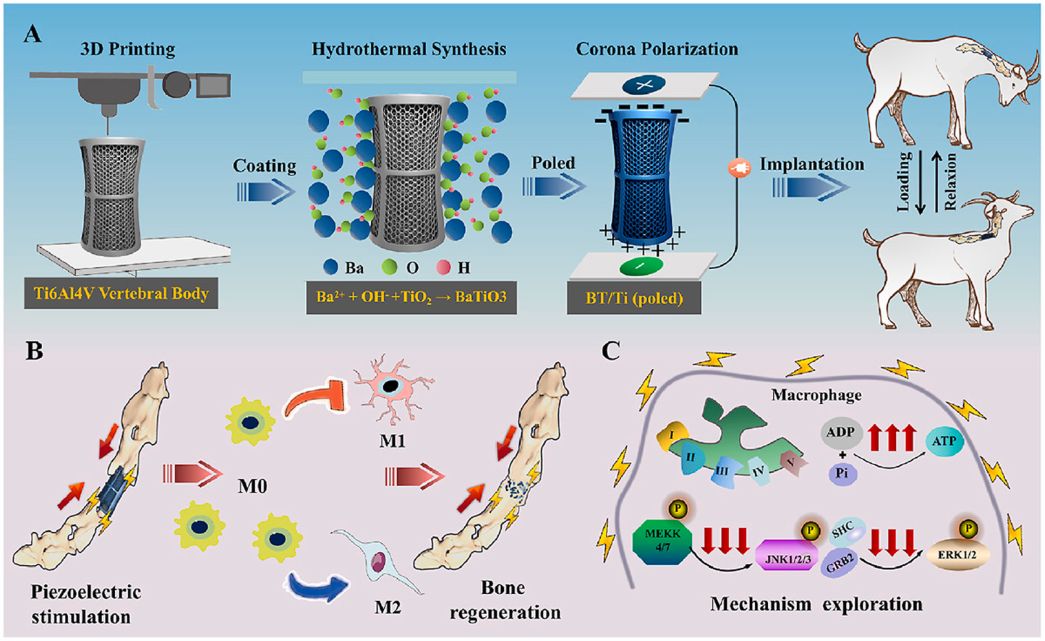
Graphical abstract
Generation of recombinant antibodies by mammalian expression system for detecting S-metolachlor in environmental waters
Abstract:
S-metolachlor (S-MET) is one of the most widely produced and applied herbicides in China. Owing to its chemical properties, it tends to persist in soil and easily contaminates surface and groundwater through leaching and runoff. This environmental persistence poses a serious threat to plant development and, through the food chain, to human health.
To address the limitations of current detection technologies and meet the growing demand for high-efficiency analytical tools, the researchers employed a mammalian expression system to generate recombinant antibodies targeting S-MET.
Building on the successful expression of these antibodies, they established a sensitive immunoassay for monitoring S-MET residues in various environmental water samples. The icELISA results showed that the recombinant antibodies retained the sensitivity, specificity, and biological activity of the original monoclonal antibodies, delivering accurate and reproducible detection in river water, agricultural runoff, and tap water. View details>>
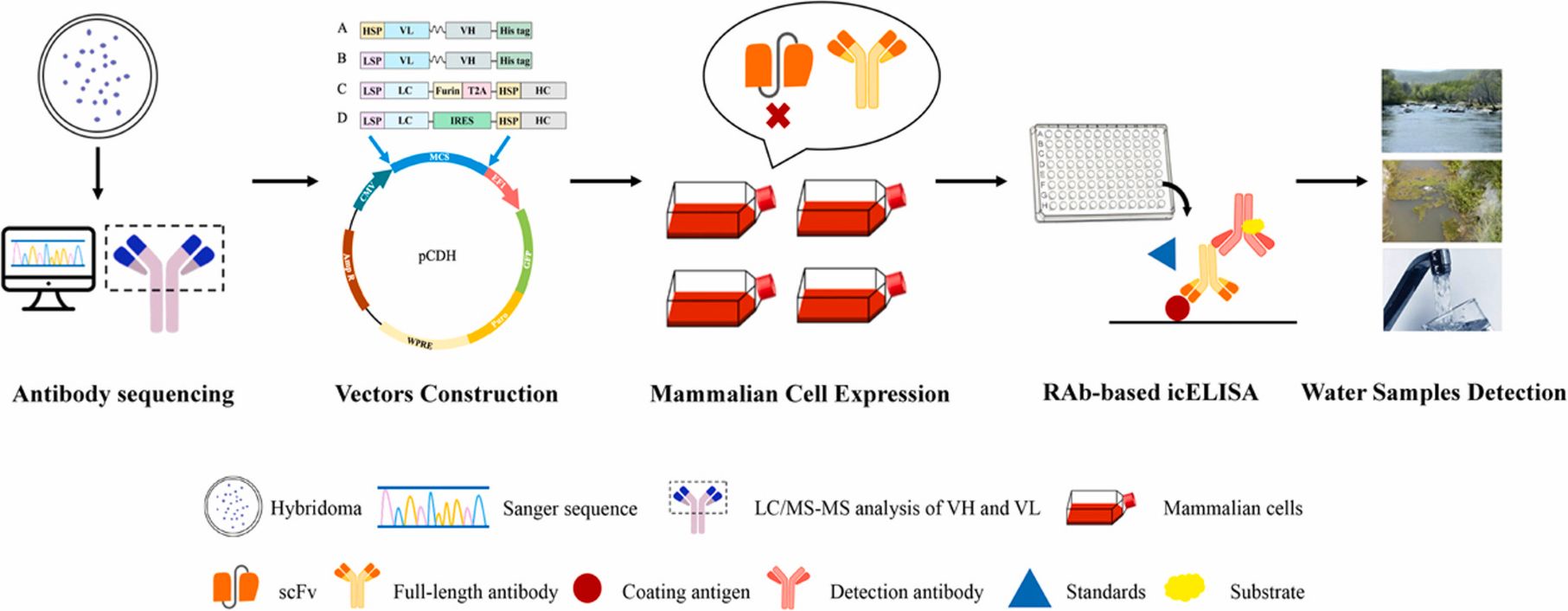
Graphical abstract
Dumbbell probe initiated multi-rolling circle amplification assisted CRISPR/Cas12a for highly sensitive detection of clinical microRNA
Abstract:
MicroRNAs (miRNAs) are a class of small non-coding RNA molecules that regulate gene expression by interacting with the mRNAs of target genes. Given their crucial role in the development and progression of various diseases, miRNAs have emerged as promising biomarkers for clinical diagnostics.
In this study, researchers established a novel detection platform, termed DBmRCA, which combines dumbbell probe-initiated multi-rolling circle amplification with the high-sensitivity signal output of CRISPR/Cas12a. This enzyme-free, isothermal method enables accurate quantification of miRNA within just 30 minutes.
Clinical validation revealed that the expression levels of miR-200a and miR-126 were significantly downregulated in lung cancer tissues, and results from DBmRCA were consistent with those obtained by conventional techniques. With its high sensitivity, rapid turnaround, and simplified workflow, the DBmRCA platform presents a reliable tool for miRNA detection and holds strong promise for early diagnosis and therapeutic monitoring of lung cancer. View details>>
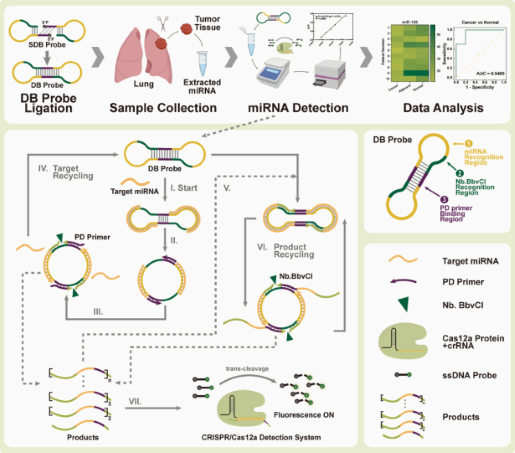
Graphical abstract




