Knock-In Cell Line
Knock-In Cell Line
Knock-in cell lines are generated by precisely inserting exogenous DNA fragments, such as fluorescent tags, reporter genes, or pathogenic mutation sequences, into specific sites of a target gene. This approach allows controlled modification of gene function and protein expression while maintaining the gene’s native regulatory environment. Compared with traditional overexpression systems, knock-in models more accurately reflect endogenous gene expression levels and regulatory mechanisms, making them valuable tools for studying gene function, disease mechanisms, and drug responses.
Leveraging EDITGENE’s optimized CRISPR/Cas9 and HDR platforms, along with efficient homology arm design and single-clone screening workflows, we provide customized knock-in cell line services. Our solutions feature high efficiency, low off-target rates, and comprehensive validation, ensuring the generation of stable and reliable cell models.Service Details
| Cell Types | Various cell types, including tumor, conventional, stem, primary, and immortalized cell lines. |
|---|---|
| Service Options | Targeted knock-in of fluorescent proteins (EGFP, Luc, mCherry, etc.) / Targeted knock-in of tag proteins (Flag, HA, Myc, HiBiT, etc.) / Targeted knock-in at specific sites of a gene of interest / Gene point mutations |
| Delivery Standard | One monoclonal stable cell line (2 cryovials, 1×10⁶ cells per vial) |
| Turnaround/Price |
As fast as 8 weeks,as low as $4500 |
Technical Principle
With over a decade of gene editing experience, EDITGENE has developed four knock-in technologies—HES-KI, CRISPaint, Bingo™, and CRISPR/HDR—capable of meeting diverse knock-in requirements. These platforms achieve both high efficiency and precision, removing barriers to the successful generation of knock-in cell lines.
| Service | Efficiency | Advantages | Applicable Scenarios | |
|---|---|---|---|---|
| CRISPR/HDR | 5-30% | Highly versatile – ideal for introducing point mutations or inserting DNA fragments | Actively dividing cells |
|
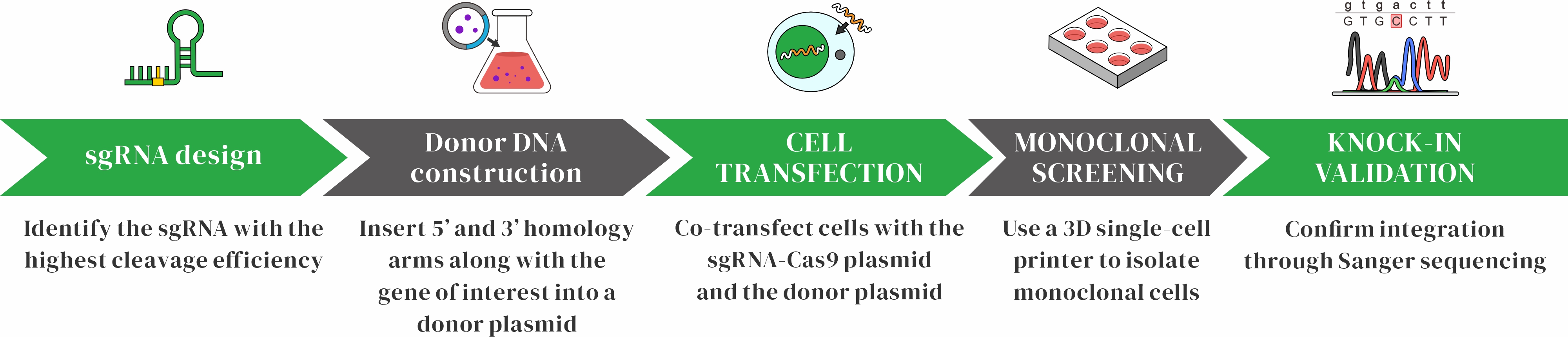
CRISPR/HDR Technology OverviewCRISPR/HDR is a gene editing approach that integrates foreign DNA through the CRISPR system and the cell’s homology-directed repair (HDR) pathway. The sgRNA guides Cas9 to a specific target site, where it introduces a double-strand break (DSB). A donor DNA template containing homology arms is then precisely inserted at the break site through HDR, enabling accurate gene knock-in.
CRISPR/HDR Technical Advantages
Highly flexibleBy adjusting the sgRNA and donor template, foreign gene sequences can be inserted at various genomic locations
Highly accurateThe HDR pathway allows precise integration of the donor DNA at the cut site, minimizing unexpected mutations (indels) at the junction
CRISPR/HDR Workflow
|
||||
| CRISPaint | 20-50% | Works independently of the cell cycle | Cells with slow or infrequent division |
|
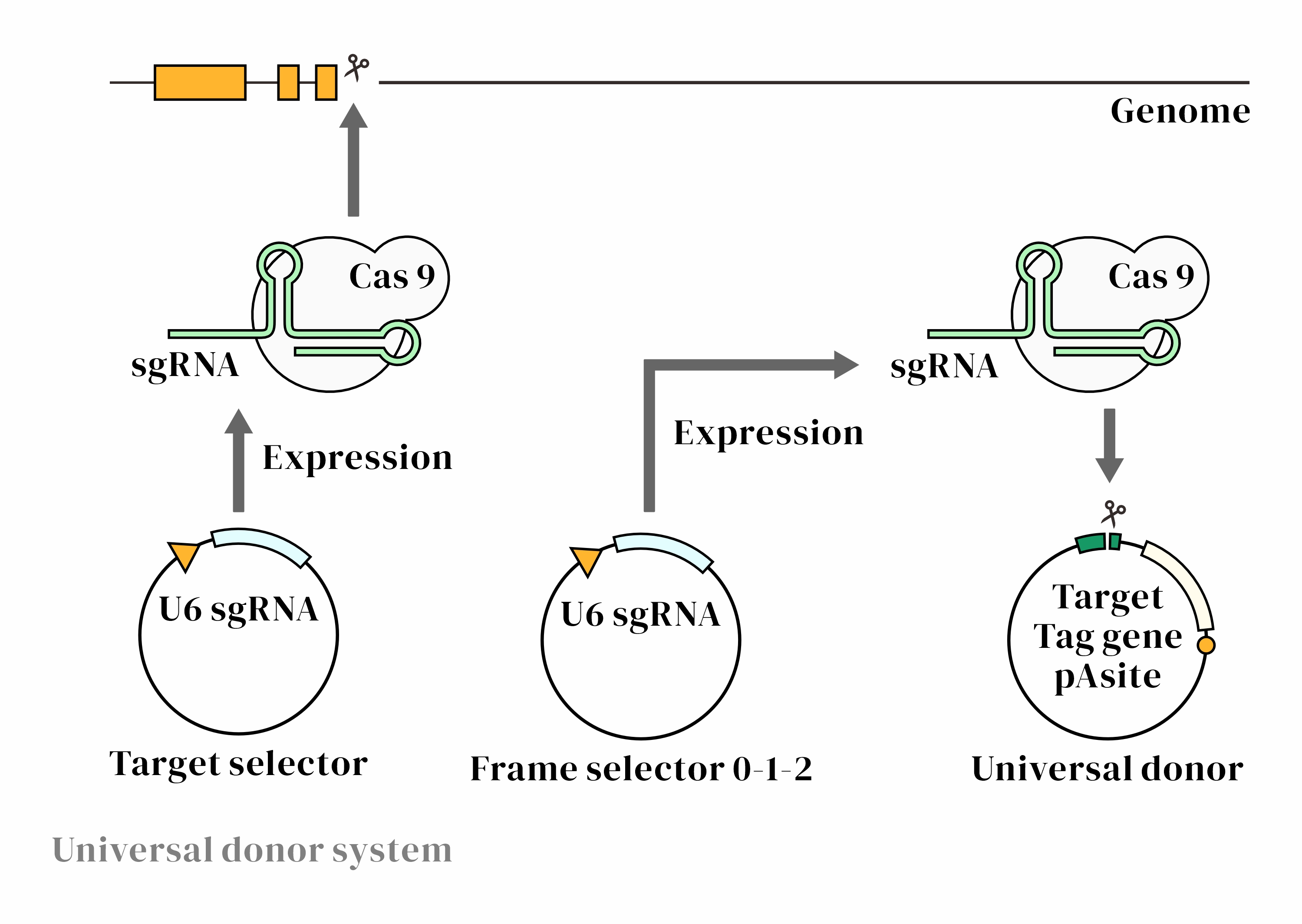
CRISPaint Technology OverviewCRISPaint is a gene knock-in technique based on the CRISPR-Cas9 system. It leverages the cell's own non-homologous end joining (NHEJ) repair mechanism to insert exogenous DNA fragments at the specified C-terminal position of a target gene, without relying on homologous recombination (HDR). The CRISPaint design is highly flexible, making it suitable for various gene function studies and genome editing applications.1. CRISPR-Cas9-Mediated Double-Strand Break (DSB)
3. Non-Homologous End Joining (NHEJ) 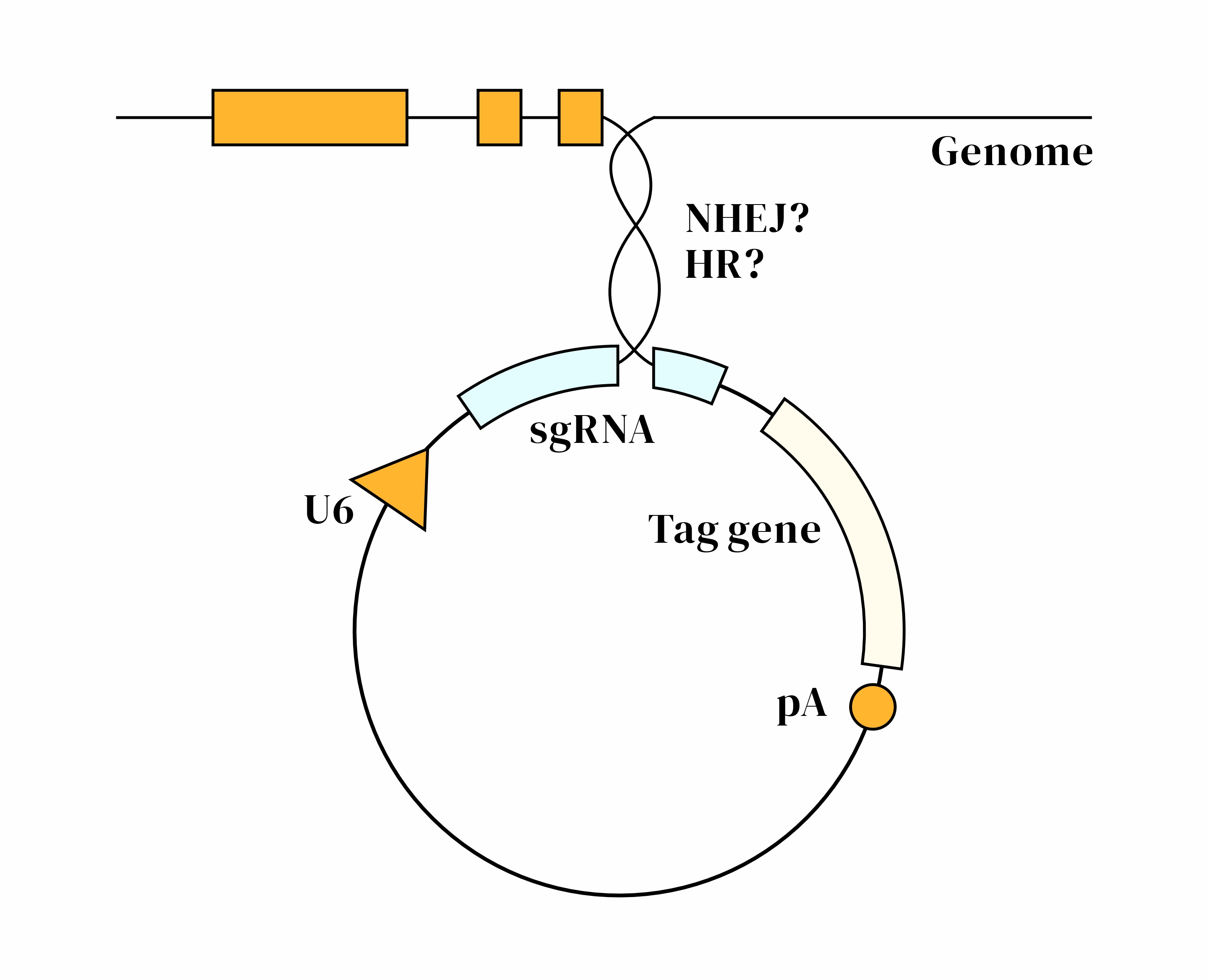
CRISPaint Technical AdvantagesHigh EfficiencyNHEJ-mediated gene knock-in requires no homology arms, achieving an insertion efficiency of up to 50%
Wide ApplicabilityIndependent of cell division, suitable for various cell types
FlexibilityThe universal donor plasmid contains three different Donor-sgRNA target sequences, allowing flexible selection of Frame sgRNAs for correct gene ORF integration
CRISPaint Experimental Workflow
|
||||
| BingoPE™ | 30-80% | No need for double-strand breaks or donor templates – low off-target risk | Precise small-fragment knock-ins or single-base corrections |
|
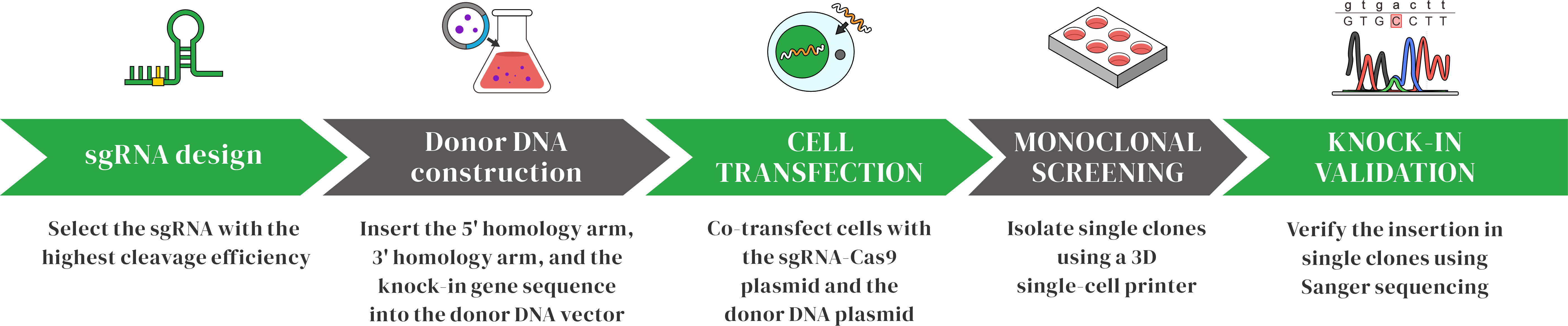
Bingo™ Technology OverviewBingo™ is a gene editing technology based on Prime Editing-mediated small fragment knock-in, without introducing double-strand breaks (DSBs). The method uses a Cas9 nickase fused to a reverse transcriptase (Cas9n-RT), guided by a prime editing guide RNA (pegRNA) to the target site. Cas9n creates a single-strand nick, and the reverse transcriptase uses the pegRNA template to synthesize the desired DNA sequence at that site, achieving accurate gene insertion.
Bingo™ Technical AdvantagesSaferPE technology edits the genome via single-strand nicking, avoiding the instability associated with DSBs and significantly improving overall safety.
Highly preciseThe pegRNA contains detailed edit instructions, enabling accurate fragment insertion and minimizing off-target or unintended edits.
VersatileSupports insertion of small DNA sequences such as stop codons or tag sequences, making it suitable for a wide range of genome editing applications.
Bingo™ Experimental Workflow
|
||||
| HES-KI | 40%-90% | Built-in screening system ensures efficient knock-in | C-terminal tagging at essential gene loci |
|
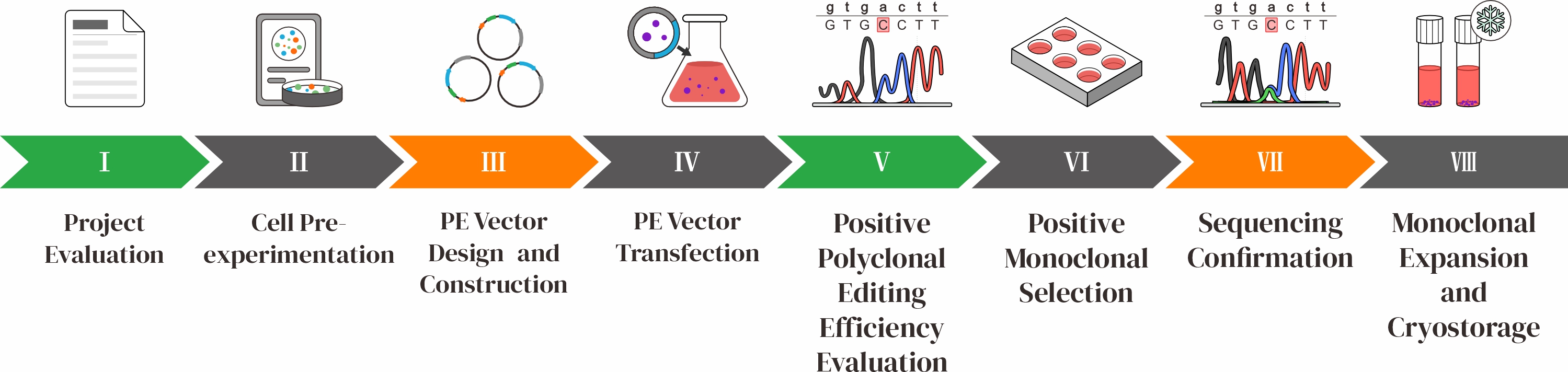
HES-KI Technology OverviewHES-KI (Hotspot-Exon-Selection Knock-In) is a novel knock-in strategy targeting the C-terminal exon region of essential genes using CRISPR editing. A custom knock-in template is designed so that successful integration restores the function of the essential gene while incorporating the desired transgene. In contrast, cells with failed knock-in—such as those with indels caused by NHEJ—lose essential gene function and cannot survive. This enables effective enrichment of correctly edited cells through a built-in positive selection mechanism. HES-KI AdvantagesHigh EfficiencyThe positive selection mechanism significantly boosts knock-in efficiency—reaching up to 68% in various cell types—far surpassing traditional methods by reducing non-specific integration
Stable and Uniform ExpressionKnock-in monoclonal cell lines show consistent and stable expression of the target gene
Multiplex Gene IntegrationSupports simultaneous integration of multiple genes. For example, multiple CAR genes can be inserted to target various tumor antigens and minimize tumor immune escape
Tunable ExpressionBy selecting different essential genes as integration sites, the expression level of the transgene can be flexibly modulated
HES-KI Technical Workflow
|
||||
Case Study
EGFP Gene Knock-in Mediated by AsCas12a Using the HES-KI System
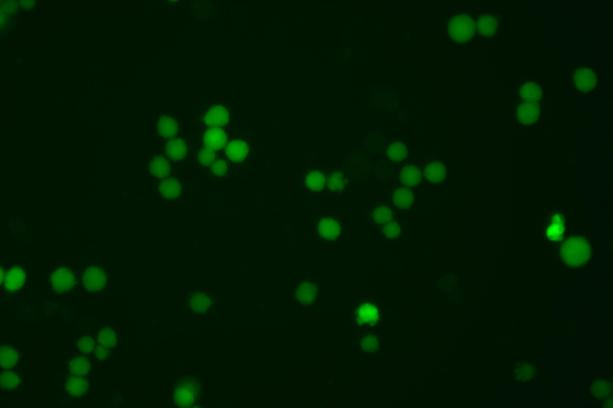
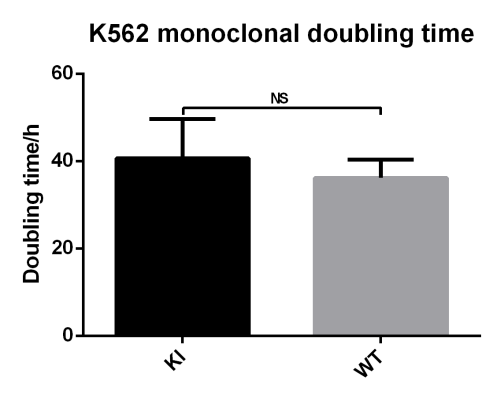
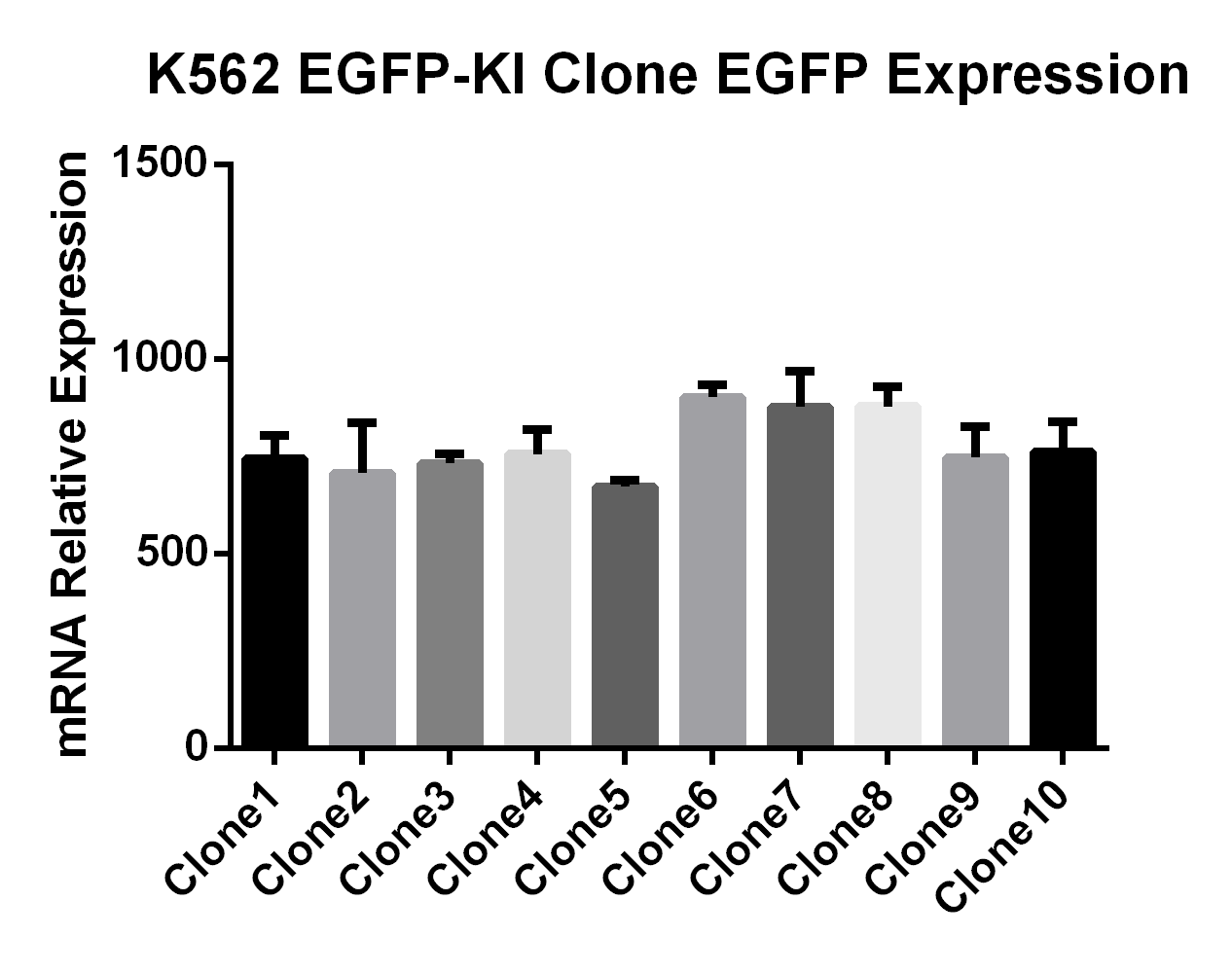
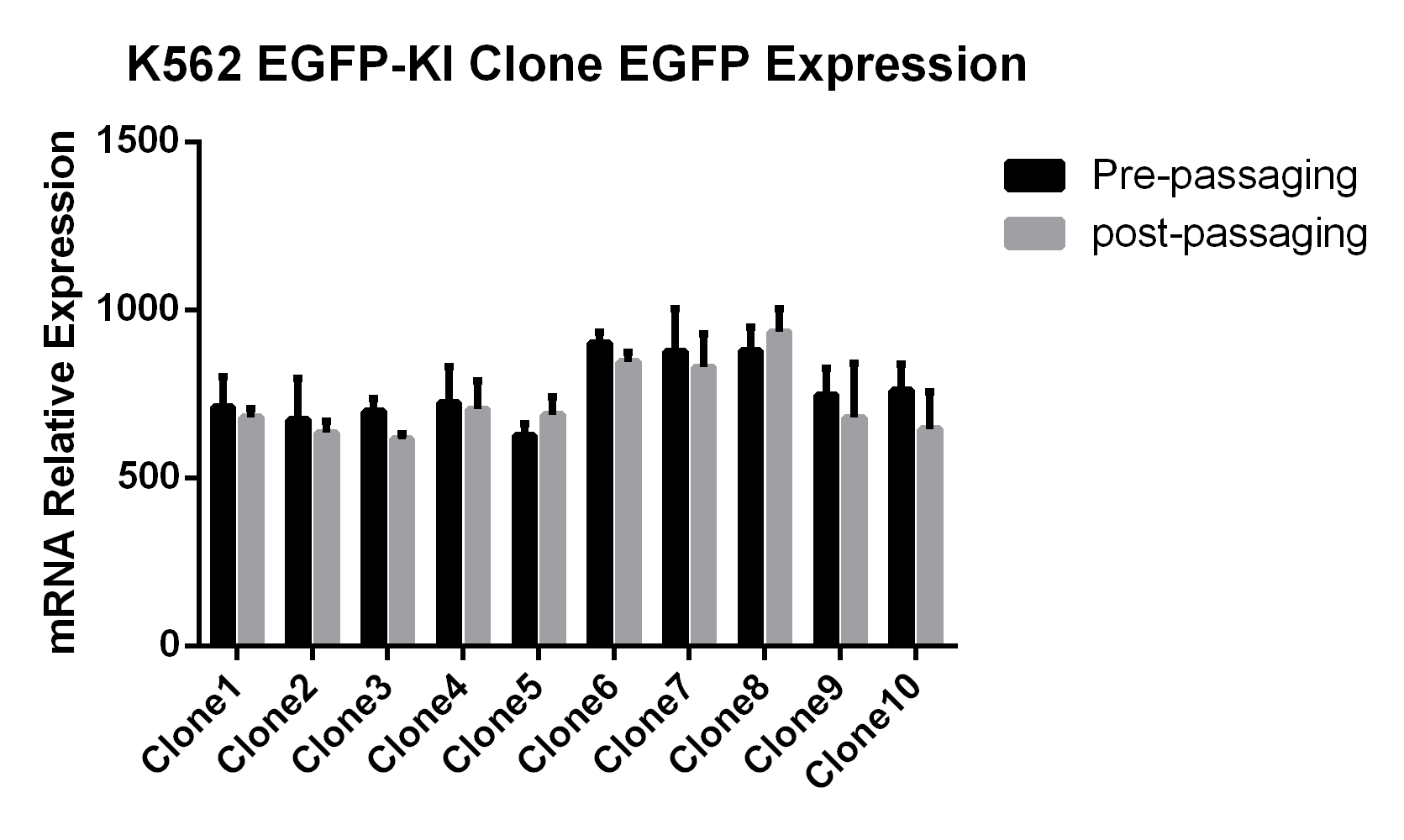
CRISPR/Cas9-Mediated EGFP Knock-In Based on the HES-KI System
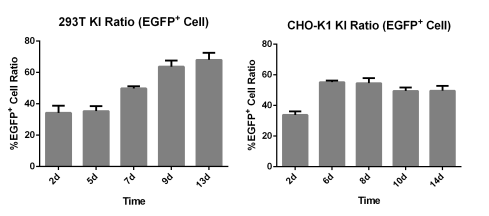
| 293T | CHO-K1 |
|---|---|
 |
 |
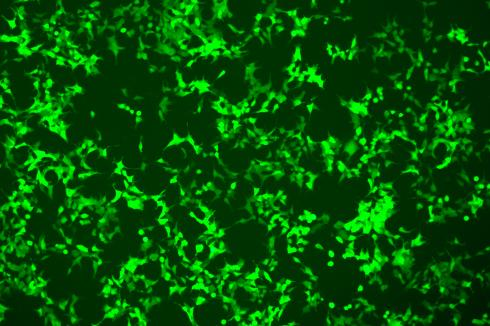 |
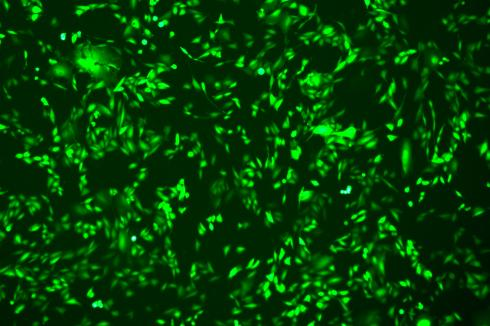 |
Generation of Base Insertion Cell Lines in Hela Cells Using Prime Editing
| Cell type | cell pool editing efficiency | Sequencing peak profile | Number of single clones selected | Number of homozygous single clones |
|---|---|---|---|---|
| Hela | 64% | WT:TCCGCTACCACCAATGCCTAATGCATTTGG MT:TCCGCTACCACCAATGCTAATAACTAATGCATTTGG 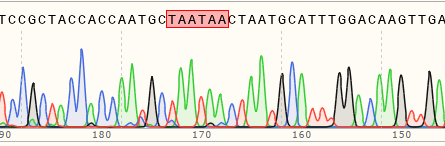 |
11 | 1 |
Advantage and Characteristic

Optimazied Strategy

Optimazied Strategy

Optimazied Strategy

Optimazied Strategy
Reference Materials
Enhancing CRISPR-mediated homology-directed repair (HDR) efficiency through cell cycle synchronization
This study explores a method to enhance CRISPR-mediated HDR efficiency by synchronizing the cell cycle. Using small molecules to modulate the cell cycle, researchers achieved a 1.2- to 1.5-fold increase in knock-in efficiency across various cell lines. The study also demonstrated this approach's application in animal embryos, significantly increasing knock-in frequency in pig embryos. This technique improves knock-in success by guiding cells to an HDR-favorable cycle stage, offering a new optimization strategy for CRISPR gene editing.
Knock-in strategy based on CLASH technology
The CLASH (Cas9-Linked Adaptor Synthesis for Homology-directed repair) technology enables efficient large-scale gene knock-in for cell engineering. This method combines the Cas9 protein and adaptor synthesis, allowing parallel knock-in across various cell types. By providing specific adaptors during the DNA repair process, it significantly enhances homology-directed repair (HDR) efficiency, thereby increasing knock-in success rates. This technology shows great potential in cell engineering and gene editing, especially for complex bioengineering applications requiring multi-gene modifications.
Selected Customer Resources
Deep whole-genome analysis of 494 hepatocellular carcinomas
Abstract:
To date, more than half of global hepatocellular carcinoma (HCC) cases occur in China, yet comprehensive whole-genome analyses focusing on HBV-related HCC within the Chinese population remain scarce. To address this challenge, researchers initiated the China Liver Cancer Atlas (CLCA) project, aiming to conduct large-scale whole-genome sequencing to unravel the unique pathogenic mechanisms and evolutionary trajectories of HCC in China.
The researchers performed deep whole-genome sequencing on 494 HCC tumor samples, with an average depth of 120×, alongside matched blood controls, providing a detailed genomic landscape of HBV-associated HCC. Beyond confirming well-known coding driver genes such as TP53 and CTNNB1, the study identified six novel coding drivers—including FGA—and 31 non-coding driver genes.
Additionally, the research uncovered five new mutational signatures, including SBS_H8, and characterized the presence of extrachromosomal circular DNA (ecDNA) formed via HBV integration, which contributes to oncogene amplification and overexpression. Functional validation experiments demonstrated that mutations in genes such as FGA, PPP1R12B, and KCNJ12 significantly enhance HCC cell proliferation, migration, and invasion.
These findings not only deepen our insights into the genomics of HCC, but also open up new potential targets for diagnosis and therapy. View details>>
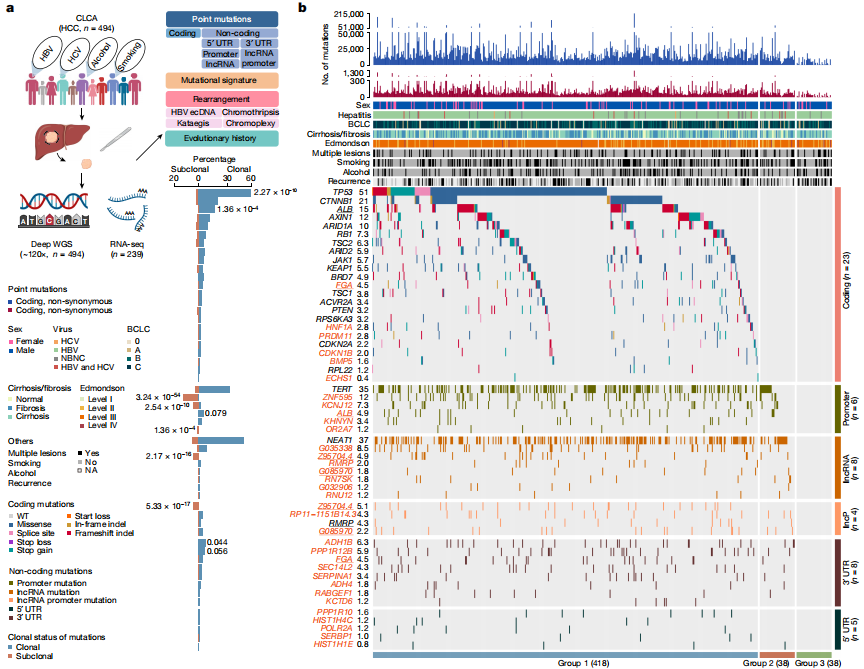
Candidate driver landscape
Targeted Macrophage CRISPR-Cas13 mRNA Editing in Immunotherapy for Tendon Injury
Abstract:
During the acute inflammatory phase of tendon injury, excessive activation of macrophages leads to the overexpression of SPP1, which encodes osteopontin (OPN), thereby impairing tissue regeneration. The CRISPR-Cas13 system holds great promise for tissue repair due to its unique RNA editing and rapid degradation capabilities; however, its application has been limited by the lack of efficient delivery methods.
To address this, the researchers systematically screened various cationic polymers targeting macrophages and developed a nanocluster carrier capable of efficiently delivering Cas13 ribonucleoprotein complexes (Cas13 RNPs) into macrophages. Utilizing a reactive oxygen species (ROS)-responsive release mechanism, this system specifically suppresses the overexpression of SPP1 in macrophages within the acute inflammatory microenvironment of tendon injury.
Experimental results demonstrated that this targeted delivery strategy significantly reduced the population of SPP1-overexpressing macrophages induced by injury, inhibited fibroblast activation, and alleviated peritendinous adhesion formation. Furthermore, the study elucidated that SPP1 promotes fibroblast activation and migration through the CD44/AKT signaling pathway, and that inhibiting this pathway effectively mitigates adhesion formation following tendon injury. View details>>
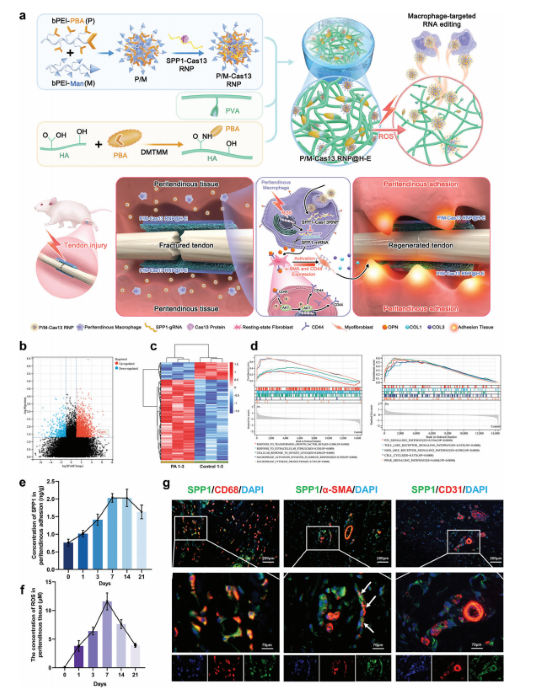
Schematic diagram illustrating immune microenvironment-activated mRNA editing strategies of macrophages for PA therapy
Electrical stimulation of piezoelectric BaTiO3 coated Ti6Al4V scaffolds promotes anti-inflammatory polarization of macrophage and bone repair via MAPK/JNK inhibition and OXPHOS activation
Abstract:
Spinal cord injury (SCI) is a severe disabling condition that causes permanent loss of sensory, autonomic, and motor functions. While stem cell therapies, particularly mesenchymal stem cells (MSCs), show great promise for SCI treatment, their limited regenerative capacity restricts their application in tissue repair. The researchers observed that extracellular vesicles derived from antler bud progenitor cells (EVsABPC) may carry bioactive signals that promote tissue regeneration. Accordingly, they isolated and engineered EVs from ABPCs for SCI therapeutic investigation.
The study found that EVsABPC significantly enhanced neural stem cell (NSC) proliferation, promoted axonal growth, reduced neuronal apoptosis, and modulated inflammation by shifting macrophage polarization from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype. Moreover, engineered EVsABPC modified with cell-penetrating peptides demonstrated improved targeting to the SCI lesion site, markedly enhancing neural regeneration and functional motor recovery. These findings highlight EVsABPC as a promising candidate for SCI therapy. View details>>
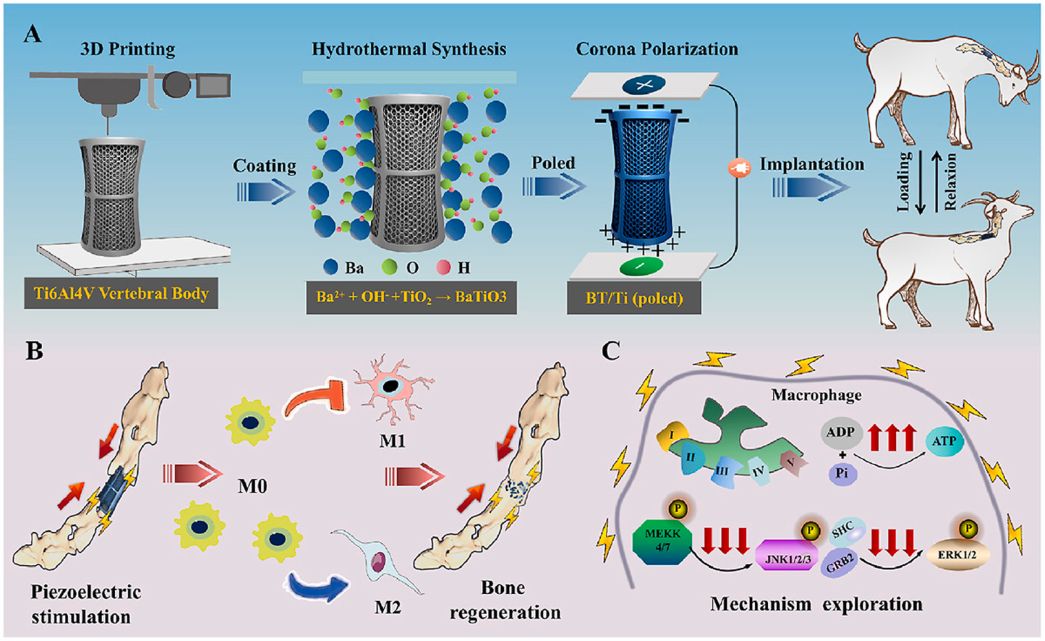
Graphical abstract
Generation of recombinant antibodies by mammalian expression system for detecting S-metolachlor in environmental waters
Abstract:
S-metolachlor (S-MET) is one of the most widely produced and applied herbicides in China. Owing to its chemical properties, it tends to persist in soil and easily contaminates surface and groundwater through leaching and runoff. This environmental persistence poses a serious threat to plant development and, through the food chain, to human health.
To address the limitations of current detection technologies and meet the growing demand for high-efficiency analytical tools, the researchers employed a mammalian expression system to generate recombinant antibodies targeting S-MET.
Building on the successful expression of these antibodies, they established a sensitive immunoassay for monitoring S-MET residues in various environmental water samples. The icELISA results showed that the recombinant antibodies retained the sensitivity, specificity, and biological activity of the original monoclonal antibodies, delivering accurate and reproducible detection in river water, agricultural runoff, and tap water. View details>>
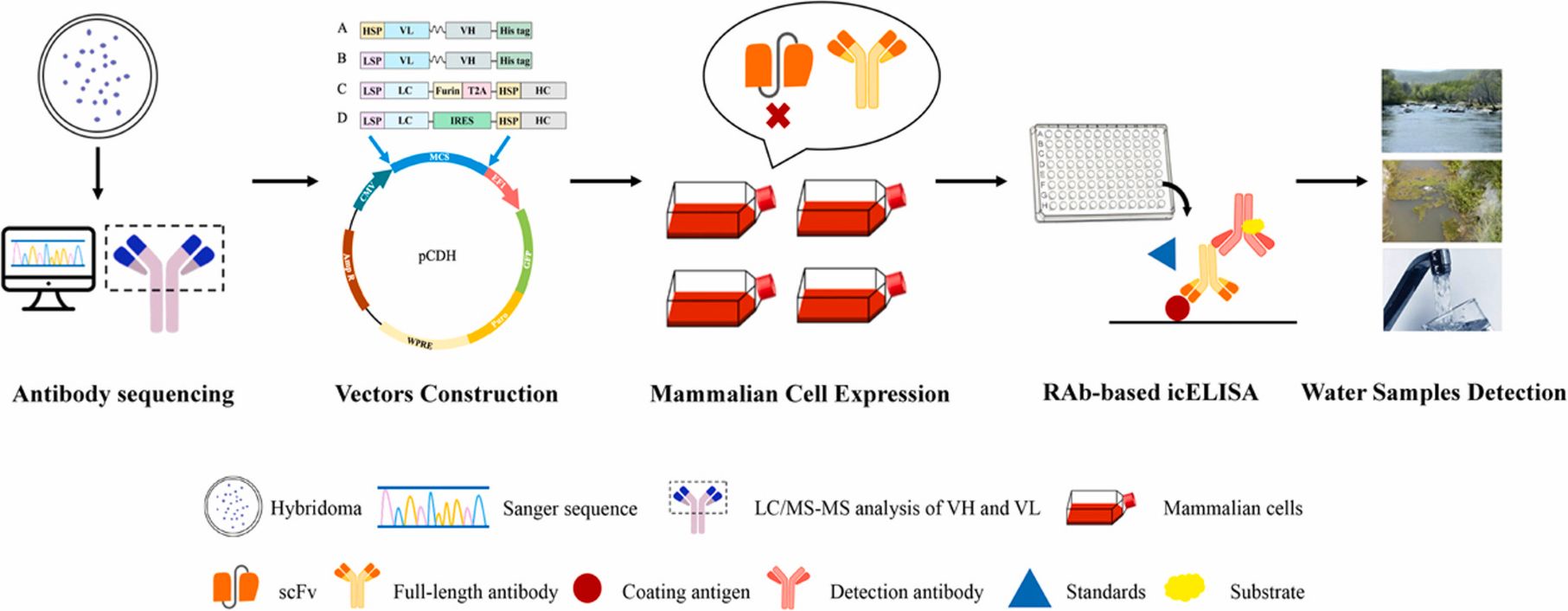
Graphical abstract
Dumbbell probe initiated multi-rolling circle amplification assisted CRISPR/Cas12a for highly sensitive detection of clinical microRNA
Abstract:
MicroRNAs (miRNAs) are a class of small non-coding RNA molecules that regulate gene expression by interacting with the mRNAs of target genes. Given their crucial role in the development and progression of various diseases, miRNAs have emerged as promising biomarkers for clinical diagnostics.
In this study, researchers established a novel detection platform, termed DBmRCA, which combines dumbbell probe-initiated multi-rolling circle amplification with the high-sensitivity signal output of CRISPR/Cas12a. This enzyme-free, isothermal method enables accurate quantification of miRNA within just 30 minutes.
Clinical validation revealed that the expression levels of miR-200a and miR-126 were significantly downregulated in lung cancer tissues, and results from DBmRCA were consistent with those obtained by conventional techniques. With its high sensitivity, rapid turnaround, and simplified workflow, the DBmRCA platform presents a reliable tool for miRNA detection and holds strong promise for early diagnosis and therapeutic monitoring of lung cancer. View details>>
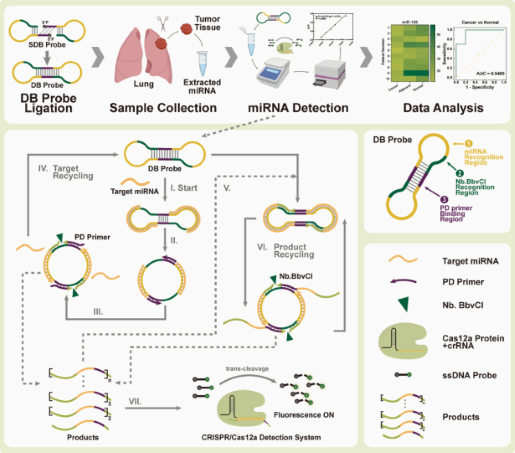
Graphical abstract