 CRISPR Library Screening
CRISPR Library Screening
Service Advantage
Service Contents and Deliverables
| Services | Description | Deliverables |
|---|---|---|
|
Custom sgRNA Library Design, Amplification, and Quality Control |
sgRNA design, sgRNA-oligo pool synthesis, vector construction, plasmid library amplification, NGS quality control |
Plasmid library: 100 µg NGS quality control report (coverage >99%, uniformity <10) |
| Cas9 Stable Cell Line Customization | Cas9 lentiviral packaging, Cas9 stable cell line construction, editing activity detection | Cas9 stable cell line (1×10^6 cells per line) |
|
Library Lentiviral Packaging |
Library viral packaging and viral titer determination by cell titration, ensuring live virus titer >1×10^6 TU/ml |
Small scale virus (total 1×10^8 TU) Medium scale virus (total 5×10^8 TU) Large scale virus (total 5×10^9 TU) Virus titer report |
| Library Cell Pool Construction | Optimization of transduction conditions (MOI <0.3), library cell pool construction | Library cell pool |
| Functional Screening Assay | Customized screening services: drug screening, viral infection, bacterial infection, flow cytometry sorting, etc. | Experimental report |
| NGS Analysis | sgRNA sequencing library construction, NGS sequencing, bioinformatics analysis | Experimental report, raw NGS sequencing data |
EDI-Service Advantages
Multiple Delivery Formats
Proprietary Development
Personalized Bioinformatics Analysis
One-Stop Service
Workflow
Case Study
Advantage and Characteristic

Optimazied Strategy

Optimazied Strategy

Optimazied Strategy

Optimazied Strategy
Selected Customer Resources
Deep whole-genome analysis of 494 hepatocellular carcinomas
Abstract:
To date, more than half of global hepatocellular carcinoma (HCC) cases occur in China, yet comprehensive whole-genome analyses focusing on HBV-related HCC within the Chinese population remain scarce. To address this challenge, researchers initiated the China Liver Cancer Atlas (CLCA) project, aiming to conduct large-scale whole-genome sequencing to unravel the unique pathogenic mechanisms and evolutionary trajectories of HCC in China.
The researchers performed deep whole-genome sequencing on 494 HCC tumor samples, with an average depth of 120×, alongside matched blood controls, providing a detailed genomic landscape of HBV-associated HCC. Beyond confirming well-known coding driver genes such as TP53 and CTNNB1, the study identified six novel coding drivers—including FGA—and 31 non-coding driver genes.
Additionally, the research uncovered five new mutational signatures, including SBS_H8, and characterized the presence of extrachromosomal circular DNA (ecDNA) formed via HBV integration, which contributes to oncogene amplification and overexpression. Functional validation experiments demonstrated that mutations in genes such as FGA, PPP1R12B, and KCNJ12 significantly enhance HCC cell proliferation, migration, and invasion.
These findings not only deepen our insights into the genomics of HCC, but also open up new potential targets for diagnosis and therapy. View details>>
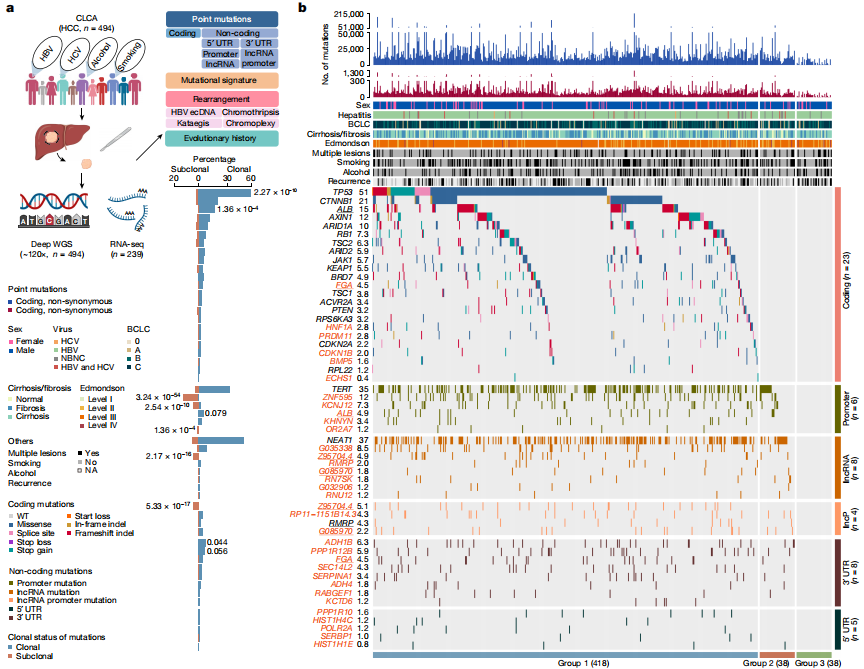
Candidate driver landscape
Targeted Macrophage CRISPR-Cas13 mRNA Editing in Immunotherapy for Tendon Injury
Abstract:
During the acute inflammatory phase of tendon injury, excessive activation of macrophages leads to the overexpression of SPP1, which encodes osteopontin (OPN), thereby impairing tissue regeneration. The CRISPR-Cas13 system holds great promise for tissue repair due to its unique RNA editing and rapid degradation capabilities; however, its application has been limited by the lack of efficient delivery methods.
To address this, the researchers systematically screened various cationic polymers targeting macrophages and developed a nanocluster carrier capable of efficiently delivering Cas13 ribonucleoprotein complexes (Cas13 RNPs) into macrophages. Utilizing a reactive oxygen species (ROS)-responsive release mechanism, this system specifically suppresses the overexpression of SPP1 in macrophages within the acute inflammatory microenvironment of tendon injury.
Experimental results demonstrated that this targeted delivery strategy significantly reduced the population of SPP1-overexpressing macrophages induced by injury, inhibited fibroblast activation, and alleviated peritendinous adhesion formation. Furthermore, the study elucidated that SPP1 promotes fibroblast activation and migration through the CD44/AKT signaling pathway, and that inhibiting this pathway effectively mitigates adhesion formation following tendon injury. View details>>
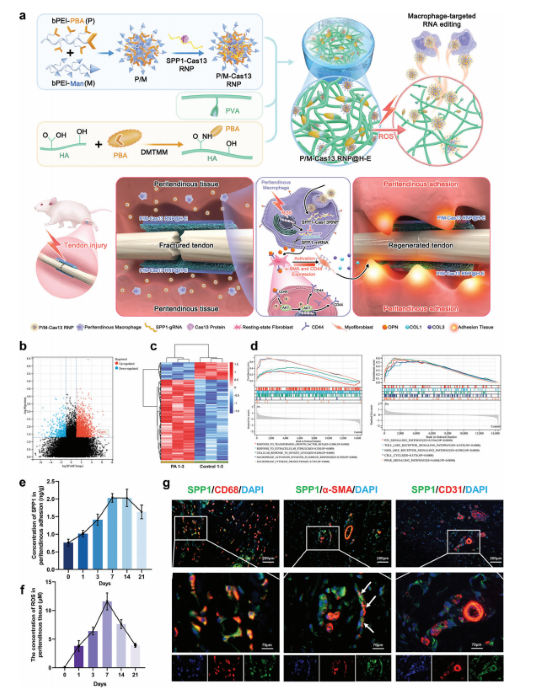
Schematic diagram illustrating immune microenvironment-activated mRNA editing strategies of macrophages for PA therapy
Electrical stimulation of piezoelectric BaTiO3 coated Ti6Al4V scaffolds promotes anti-inflammatory polarization of macrophage and bone repair via MAPK/JNK inhibition and OXPHOS activation
Abstract:
Spinal cord injury (SCI) is a severe disabling condition that causes permanent loss of sensory, autonomic, and motor functions. While stem cell therapies, particularly mesenchymal stem cells (MSCs), show great promise for SCI treatment, their limited regenerative capacity restricts their application in tissue repair. The researchers observed that extracellular vesicles derived from antler bud progenitor cells (EVsABPC) may carry bioactive signals that promote tissue regeneration. Accordingly, they isolated and engineered EVs from ABPCs for SCI therapeutic investigation.
The study found that EVsABPC significantly enhanced neural stem cell (NSC) proliferation, promoted axonal growth, reduced neuronal apoptosis, and modulated inflammation by shifting macrophage polarization from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype. Moreover, engineered EVsABPC modified with cell-penetrating peptides demonstrated improved targeting to the SCI lesion site, markedly enhancing neural regeneration and functional motor recovery. These findings highlight EVsABPC as a promising candidate for SCI therapy. View details>>
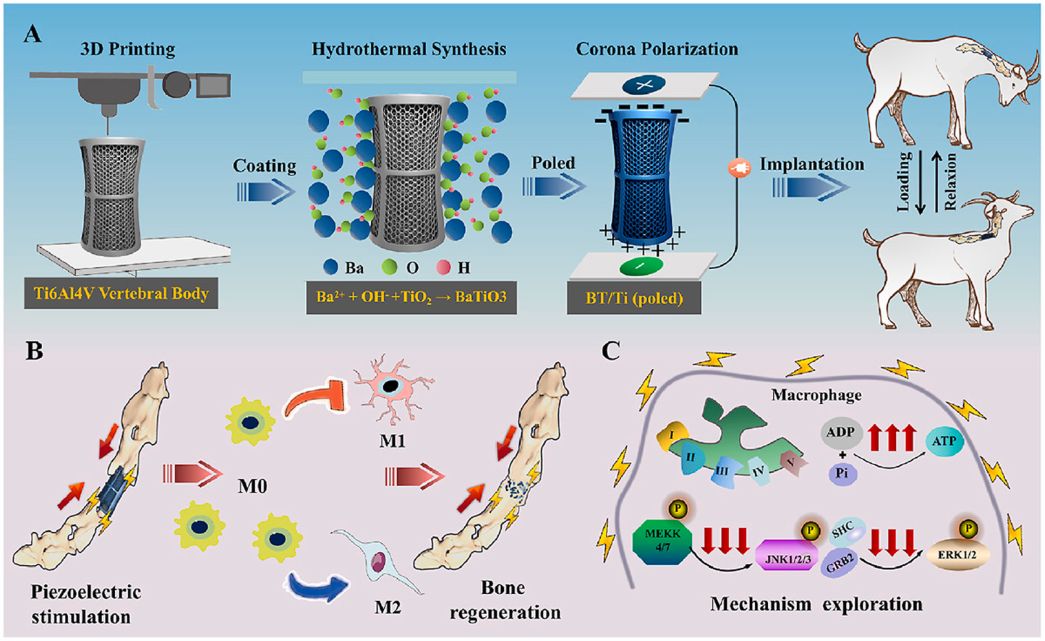
Graphical abstract
Generation of recombinant antibodies by mammalian expression system for detecting S-metolachlor in environmental waters
Abstract:
S-metolachlor (S-MET) is one of the most widely produced and applied herbicides in China. Owing to its chemical properties, it tends to persist in soil and easily contaminates surface and groundwater through leaching and runoff. This environmental persistence poses a serious threat to plant development and, through the food chain, to human health.
To address the limitations of current detection technologies and meet the growing demand for high-efficiency analytical tools, the researchers employed a mammalian expression system to generate recombinant antibodies targeting S-MET.
Building on the successful expression of these antibodies, they established a sensitive immunoassay for monitoring S-MET residues in various environmental water samples. The icELISA results showed that the recombinant antibodies retained the sensitivity, specificity, and biological activity of the original monoclonal antibodies, delivering accurate and reproducible detection in river water, agricultural runoff, and tap water. View details>>
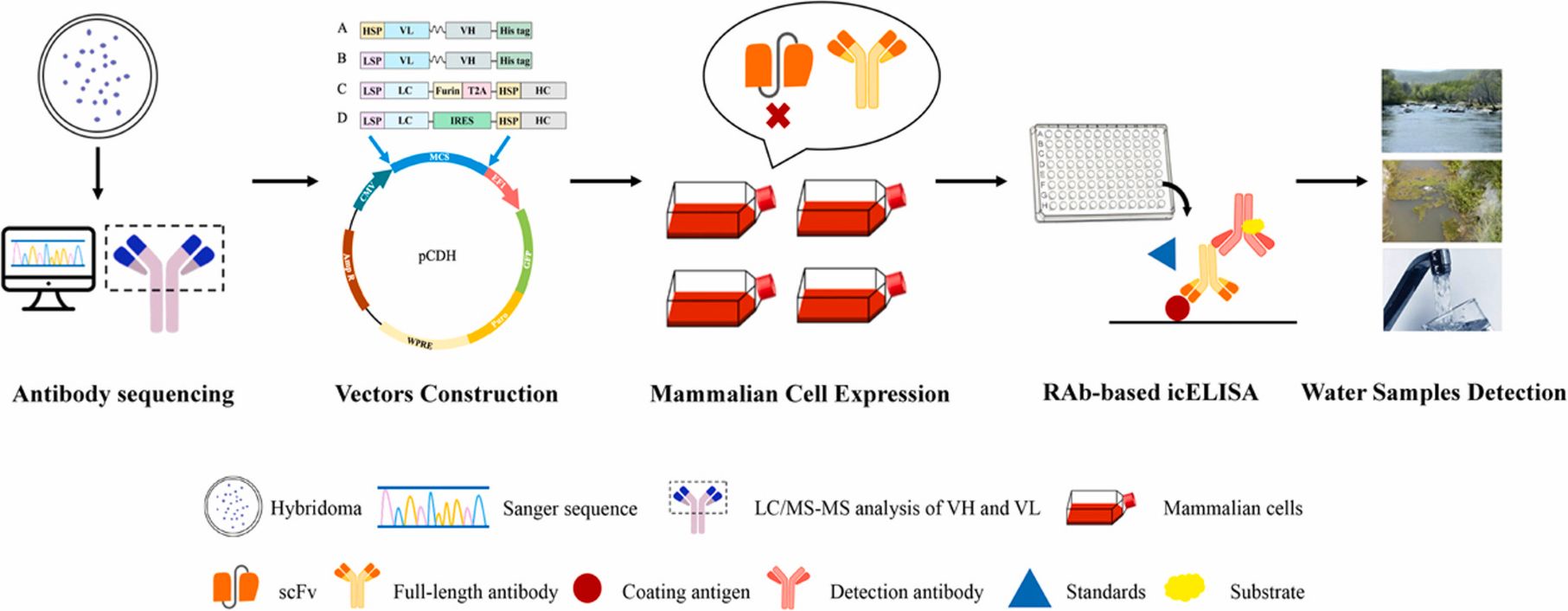
Graphical abstract
Dumbbell probe initiated multi-rolling circle amplification assisted CRISPR/Cas12a for highly sensitive detection of clinical microRNA
Abstract:
MicroRNAs (miRNAs) are a class of small non-coding RNA molecules that regulate gene expression by interacting with the mRNAs of target genes. Given their crucial role in the development and progression of various diseases, miRNAs have emerged as promising biomarkers for clinical diagnostics.
In this study, researchers established a novel detection platform, termed DBmRCA, which combines dumbbell probe-initiated multi-rolling circle amplification with the high-sensitivity signal output of CRISPR/Cas12a. This enzyme-free, isothermal method enables accurate quantification of miRNA within just 30 minutes.
Clinical validation revealed that the expression levels of miR-200a and miR-126 were significantly downregulated in lung cancer tissues, and results from DBmRCA were consistent with those obtained by conventional techniques. With its high sensitivity, rapid turnaround, and simplified workflow, the DBmRCA platform presents a reliable tool for miRNA detection and holds strong promise for early diagnosis and therapeutic monitoring of lung cancer. View details>>
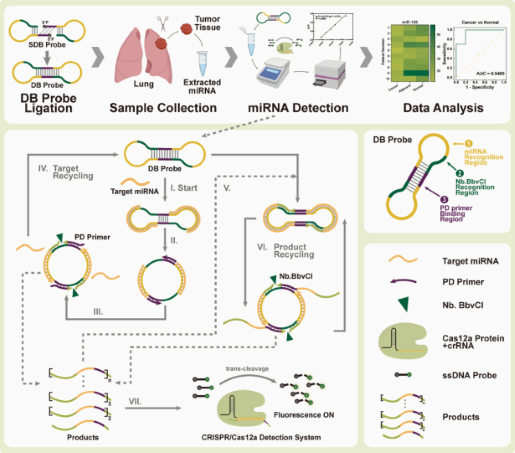
Graphical abstract
FAQ
How do I choose between a whole-genome or subgenomic CRISPR library?
How do I choose suitable cells for library screening?
1.It should align with the research objectives.
2.The genes targeted by the sgRNA library should correspond to the cell's lineage.
3.The cells should be capable of stable passaging.
4.The transfection efficiency should be high.
5.Avoid primary cells whenever possible. Primary cells cannot be stably passaged and may experience significant cell death during the library screening process, which can hinder experiment completion. If primary cells must be used for library screening, mitigating this risk can be achieved by lowering cell coverage and choosing a library with fewer gRNAs to minimize the cell pool size and shorten the experimental duration.
What is the difference between a single-plasmid system and a dual-plasmid system for library vectors?
1.Increased Editing Efficiency: The independent and stable expression of Cas9 protein and sgRNA on different vectors enhances editing efficiency.
2.Flexibility: Vectors can be designed and constructed flexibly based on experimental needs, such as loading two sgRNA expression cassettes into one vector.
3.Increased Viral Titer: By splitting into two plasmids, the load on each plasmid is reduced, facilitating viral packaging and increasing yield and titer.
4.Increased Stability: Independently constructing a stable Cas9 cell line ensures that the Cas9 expression levels and editing efficiency in each cell are approximately the same, enhancing experimental accuracy.




