[Literature Review] A “Gene Scalpel” Emerges: A New Hope for Tay-Sachs Disease Cure
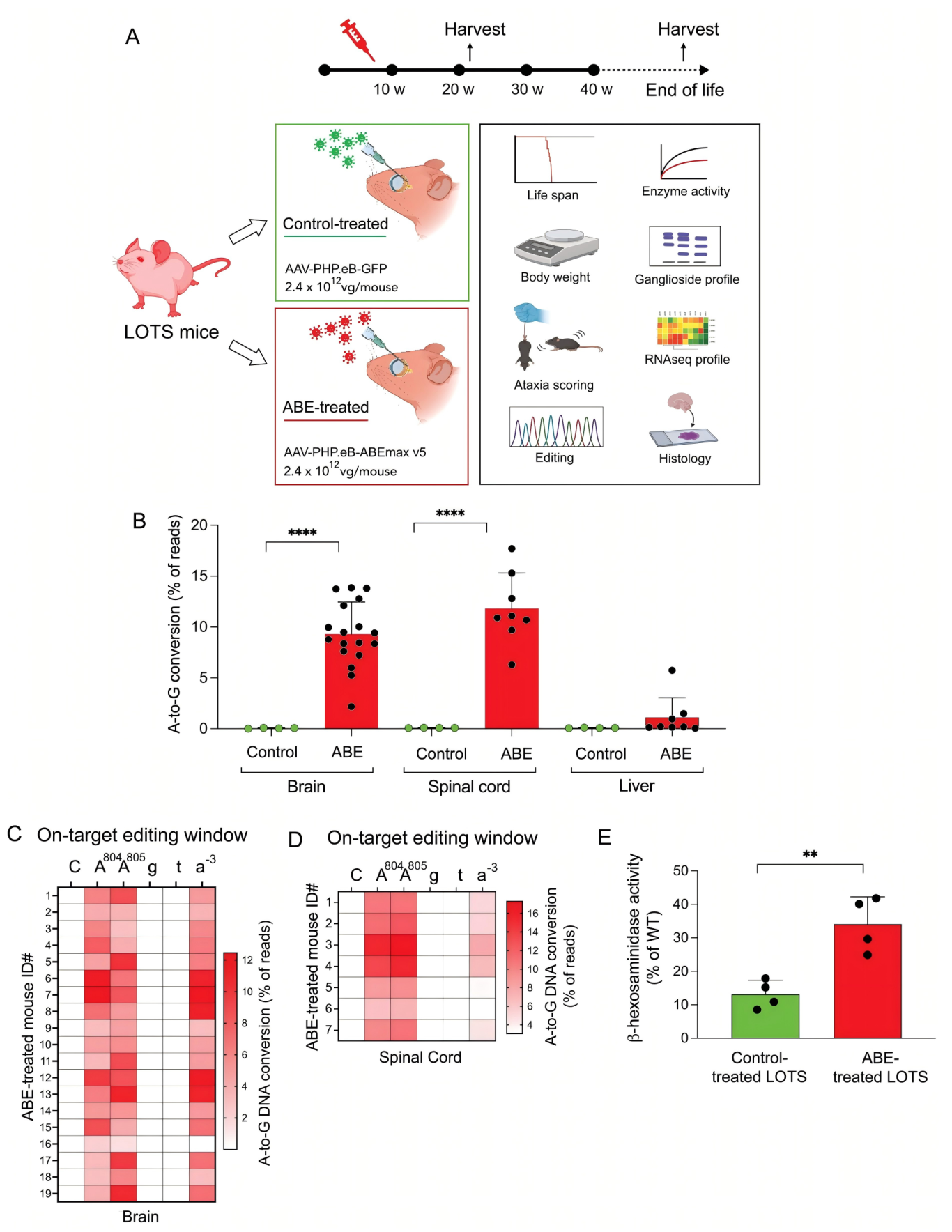
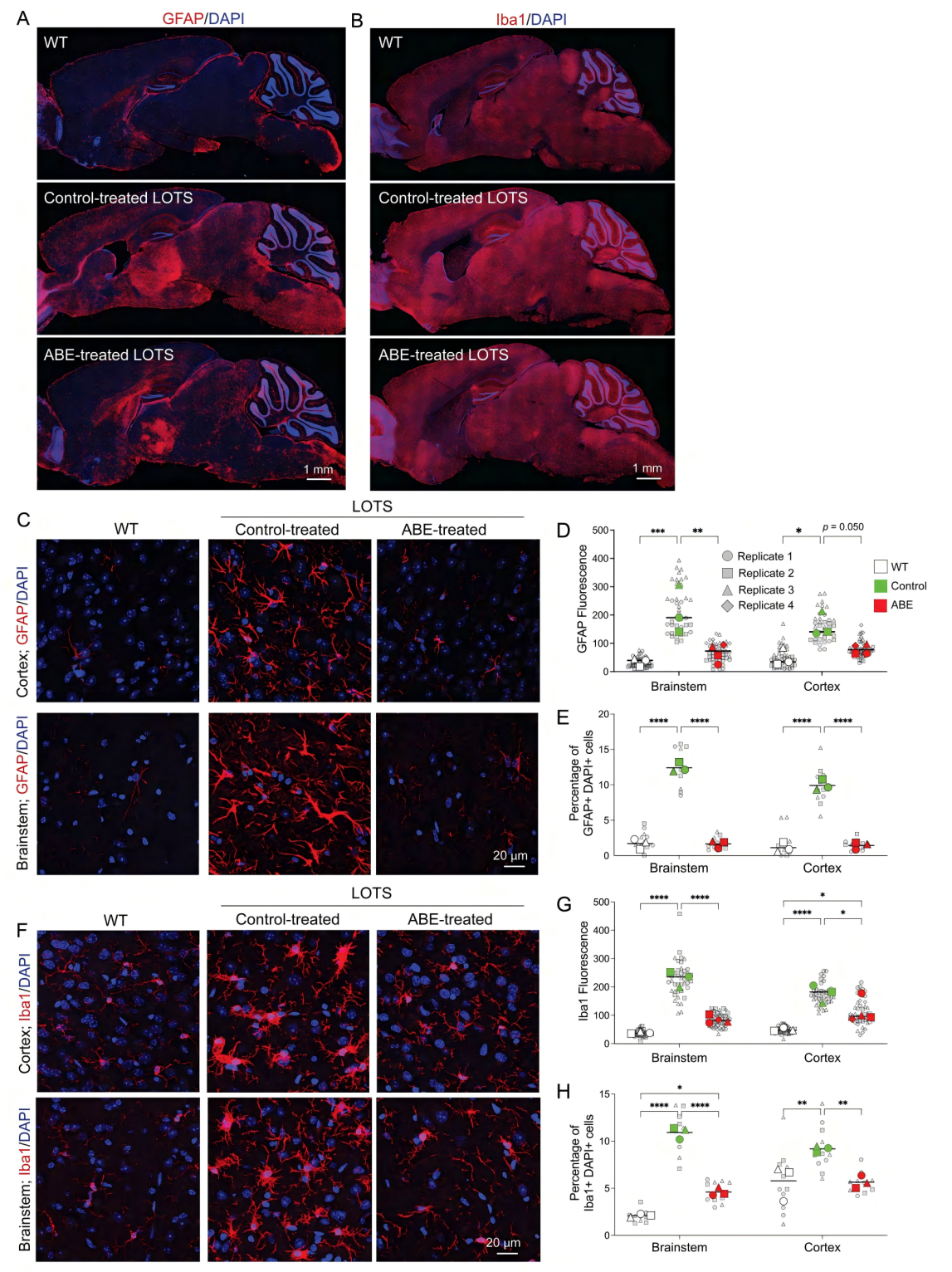
At the forefront of gene therapy, a new approach targeting neurodegenerative diseases is gaining attention. Late-onset Tay-Sachs disease (LOTS), a rare lysosomal storage disorder, is caused by a seemingly subtle point mutation in the HEXA gene—c.805G>A. Though seemingly minor, this mutation leads to the loss of β-hexosaminidase A activity, resulting in GM2 ganglioside accumulation and progressive neuronal damage. A recent study published in The Journal of Clinical Investigation (IF: 13.6), titled “CNS-targeted base editing of the major late-onset Tay-Sachs mutation alleviates disease in mice,” demonstrated the therapeutic potential of adenine base editors (ABE) guided by sgRNA. In a LOTS mouse model, researchers successfully corrected the HEXA mutation in the central nervous system, significantly improving disease symptoms and highlighting a promising path toward effective treatment. Original link: https://doi.org/10.1172/JCI183434 Spotlight 1. Precision Gene Correction with ABE: 2. Crossing the Blood-Brain Barrier with AAV-PHP.eB: 3. Extending Lifespan and Altering Disease Course: To evaluate the efficacy of the ABE system, the researchers first conducted correction experiments in fibroblasts derived from LOTS patients. Upon transduction with lentiviruses carrying both the ABE construct and LOTS-specific sgRNA, the pathogenic adenine at position c.805 was successfully converted to guanine. Sequencing results confirmed the precise base conversion, accompanied by the reappearance of the mature β-hexosaminidase A subunit and a corresponding restoration of enzyme activity. Notably, whole-genome off-target analysis using CIRCLE-seq revealed an off-target rate of less than 0.15%, demonstrating high editing precision and underscoring the potential clinical safety of this approach. To further validate the therapeutic effect in vivo, the researchers developed a customized LOTS mouse model. Using CRISPR-Cas9, they introduced the human HEXA c.805G>A mutation into the mouse genome and combined it with a Neu3 knockout background. This model successfully recapitulated key pathological features of human LOTS, including reduced enzyme activity, GM2 ganglioside accumulation in the brain, and progressive neurological symptoms, providing a robust foundation for preclinical in vivo studies. Figure 1. Construction of a LOTS mouse model for base editing One of the major challenges in this study was the efficient delivery of gene-editing tools to the central nervous system (CNS). To address this, the researchers utilized the AAV-PHP.eB serotype, known for its robust ability to cross the blood-brain barrier. Due to the relatively large size of the ABE construct, they innovatively employed a dual-AAV system in which the protein was split using an intein-mediated trans-splicing strategy. This enabled reconstitution of the full-length ABE enzyme within the target cells. Following treatment, targeted base editing of the c.805G>A mutation was achieved with correction efficiencies of 9.3% in the brain and 11.8% in the spinal cord of LOTS mice. While editing efficiency in peripheral organs such as the liver remained low, this highlighted the CNS-specific targeting advantage of the AAV-PHP.eB vector. Figure 2. Base editing corrects the pathogenic HEXA mutation in LOTS mice Neuroinflammation plays a critical role in the pathogenesis of LOTS. RNA sequencing and immunohistochemical analyses revealed that ABE treatment significantly downregulated the expression of neuroinflammation-associated genes in the brain, including activation markers of astrocytes and microglia. Immunostaining for GFAP and Iba1 showed a reduction in the number of activated glial cells, with morphology shifting from an activated to a resting state. These findings indicate that the therapy not only corrects metabolic abnormalities but also alleviates inflammatory damage. At the behavioral level, ABE-treated mice exhibited a delayed onset of weight loss and significantly lower ataxia scores compared to controls. Survival analysis demonstrated that the median lifespan of treated mice nearly doubled relative to controls, providing strong evidence that base editing therapy effectively slows disease progression and extends survival. Figure 3. Pathological changes in glial cells in the brains of LOTS mice This study opens a novel therapeutic avenue for LOTS by demonstrating the substantial potential of base editing technology in treating central nervous system disorders. However, significant challenges remain on the path from laboratory research to clinical application. These include overcoming pre-existing immunity to AAV vectors in humans, enhancing editing efficiency—particularly in critical regions such as the cerebellum—and further optimizing vector systems to reduce costs and immunogenicity. Notably, the observed phenomenon of “cross-correction,” whereby enzymes produced by edited cells are taken up by unedited cells, offers promising new strategies to improve therapeutic outcomes. With continued technological advancements, base editing holds the potential to become a universal tool for treating LOTS and other monogenic genetic diseases, bringing hope to countless patients worldwide.

Guided by a specific sgRNA, the adenine base editor (ABE) precisely targeted the pathogenic mutation in the HEXA gene. In fibroblasts derived from LOTS patients, the correction efficiency reached approximately 80%, with β-hexosaminidase A activity restored to around 50% of normal levels, with minimal off-target effects observed.
The AAV-PHP.eB vector enabled efficient delivery of ABE across the blood-brain barrier. In the LOTS mouse model, central nervous system editing successfully reduced GM2 ganglioside accumulation by nearly 90%.
ABE treatment significantly prolonged the median lifespan of LOTS mice from 27 weeks to 52.5 weeks, accompanied by a marked delay in the onset of neurological symptoms.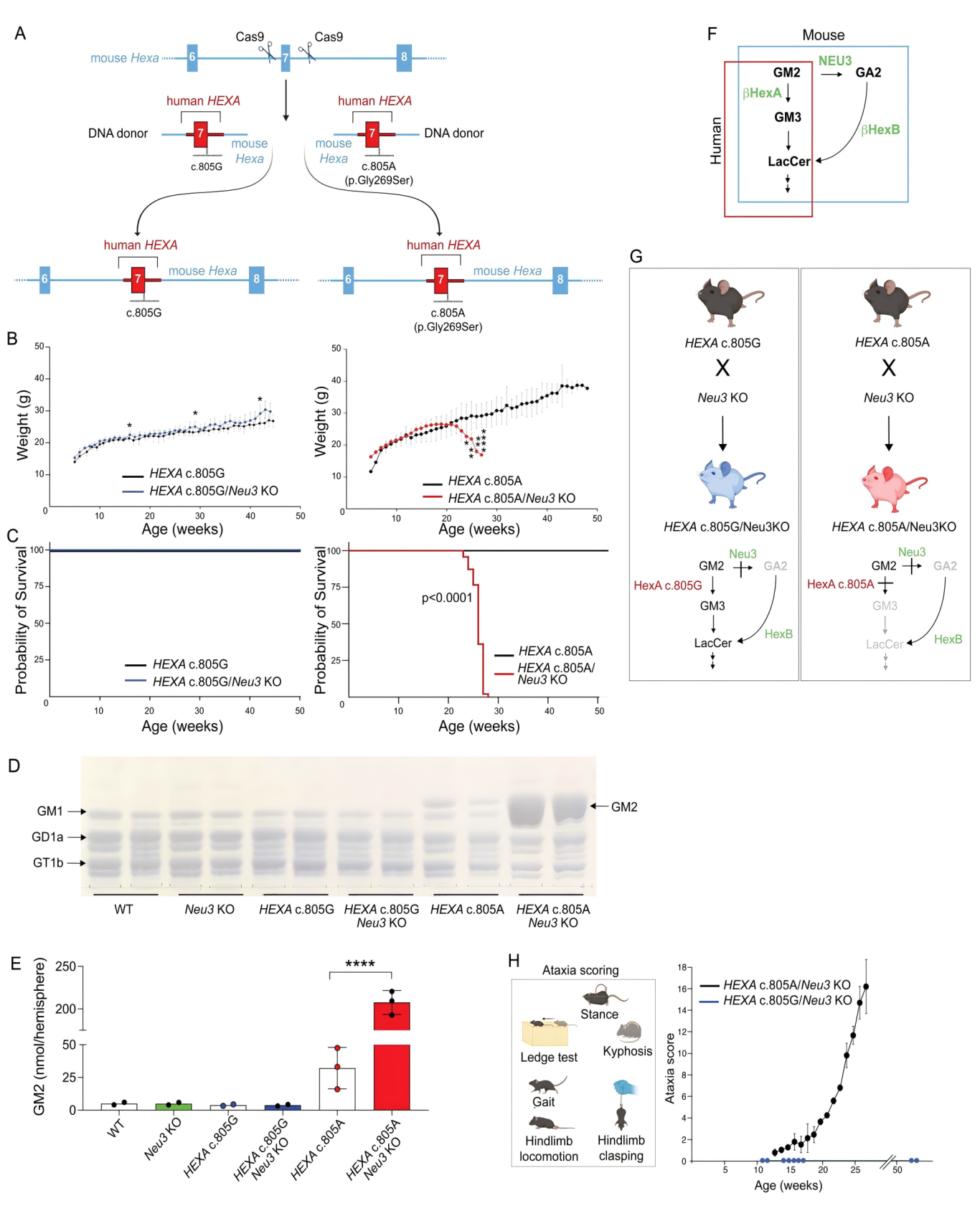















![[Literature Review] A “Gene Scalpel” Emerges: A New Hope for Tay-Sachs Disease Cure](/uploads/20250527/bL2GJjteMDvzmZys_53c82bdd67704fe0e159246934f924ee.png)

Comment (4)