[Literature Review] CRISPR Sparks Innovation: CreDiT Assay Redefines Diagnostics for Underserved Areas

The global rise in HPV-driven cancers, including cervical, head and neck cancers, and anal, is particularly pronounced in low- and middle-income countries (LMICs), where over 80% of these cervical cancer cases occur. Timely and reliable identification of high-risk HPV (hrHPV) is crucial to addressing pathology bottlenecks, as well as overcoming geographical and socioeconomic barriers to cervical cancer screening in these underserved regions.
Molecular diagnostics for hrHPV offer significantly higher sensitivity and specificity compared to traditional cytology, prompting developed countries to adopt centralized nucleic acid testing. However, the high costs and infrastructure demands of PCR-based assays limit their feasibility in LMICs, where point-of-care (POC) solutions are needed to support the "screen-and-treat" approach. Visual inspection with acetic acid (VIA) remains a low-cost alternative, but its poor accuracy highlights the urgent need for rapid, accurate, and affordable hrHPV testing in LMICs.
Original Article Link: https://doi.org/10.1038/s41467-024-50588-3
CRISPR-based detection technology has emerged as a powerful tool for nucleic acid (NA) detection due to its ability to achieve sequence-specific signal amplification. When a CRISPR-associated (Cas) protein binds to its target NA through guide RNA (gRNA), it activates as an endonuclease, amplifying signals by cleaving non-targeted signaling probes, such as single-stranded NAs tagged with fluorescent dye and quencher pairs.
Based on this mechanism, the researchers from the Center for Systems Biology at Massachusetts General Hospital Research Institute have developed CreDiT (CRISPR Enhanced Digital Testing), a point-of-care (POC) technology designed for decentralized HPV testing and HPV-related cancer screening. Their findings were published in the journal Nature Communications (IF:14.7) under the title “Empowering the on-site detection of nucleic acids by integrating CRISPR and digital signal processing”.
CreDiT integrates the Cas reaction for target NA amplification with efficient signal processing inspired by digital radio communications, offering fast, isothermal, single-tube assay with robust, noise-resistant signal detection. Aimed to detect high-risk HPV genes and oncoprotein mRNAs, CreDiT can process up to 12 samples simultaneously, accurately detects single copies of HPV DNA targets within 35 minutes, and classify high-risk HPV types in clinical cervical and anal samples. The versatility of this CRISPR-associated CreDiT assay is a valuable tool for clinical diagnostics, particularly in resource-limited settings.
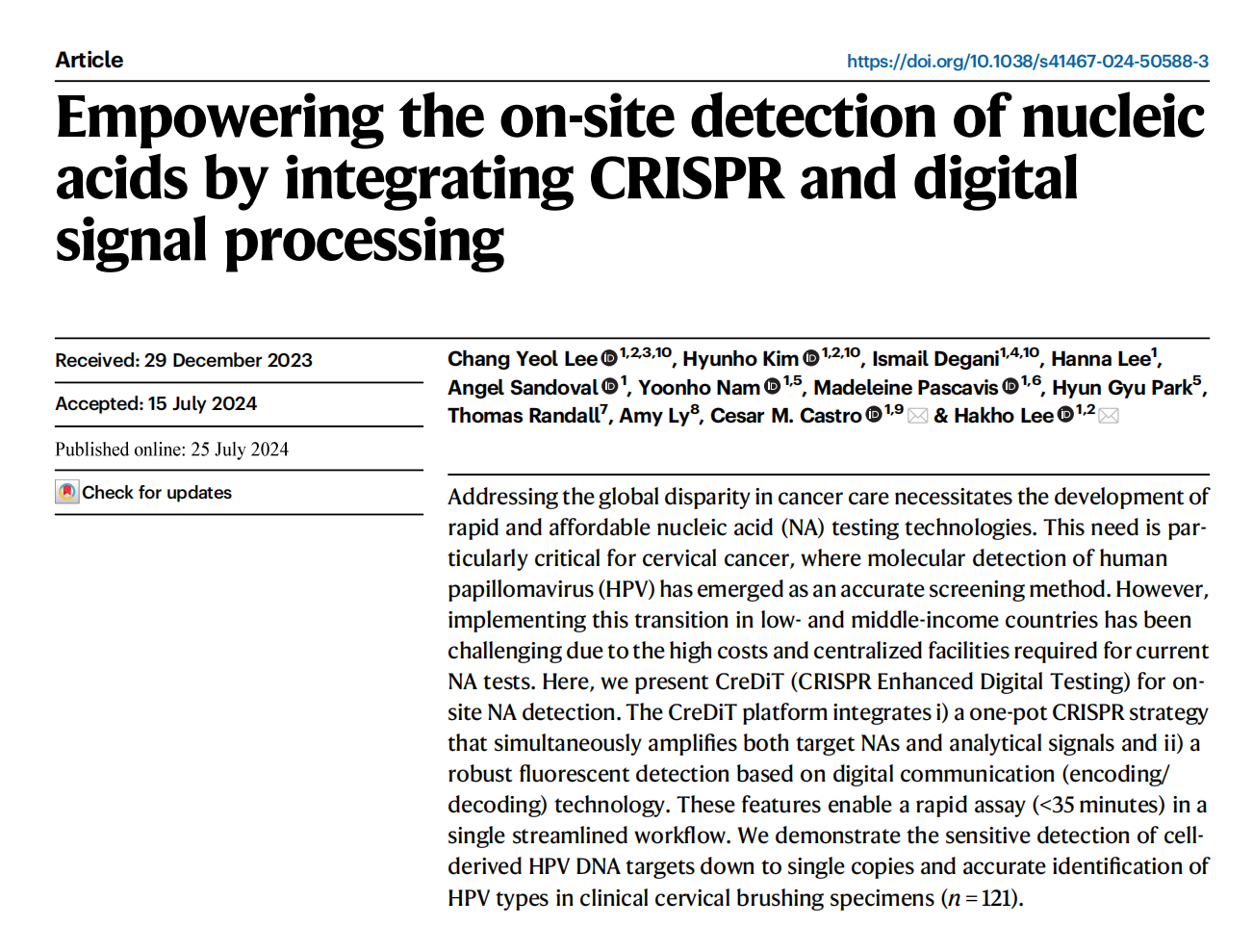
I. CreDiT platform development
As an innovative nucleic acid (NA) assay, the CreDiT assay integrates NA amplification and Cas12a-based NA recognition for rapid and sensitive detection. Activated Cas12a interferes with Recombinase Polymerase Amplification (RPA) through cis-cleavage of target DNA and trans-cleavage of RPA primers, fortunately, RPA amplification outpaces these reactions, ensuring effective target DNA RPA amplification in the CreDiT system.
This all-in-one approach eliminates the need for external intervention, and operates at a fixed temperature without adjustments. This assay concurrently amplifies target DNA and analytical signals, ensuring efficient sample processing.
To enable on-site testing, the researchers developed an automated assay system. This system was designed for multi-sample measurement, ensuring consistent signal detection, with a compact form factor of 14 × 11 × 6.5 cm3. This automated CreDiT assay holds great promise for point-of-care testing in resource-limited settings, addressing the unmet need for fast and accurate hrHPV testing, particularly in underserved regions.
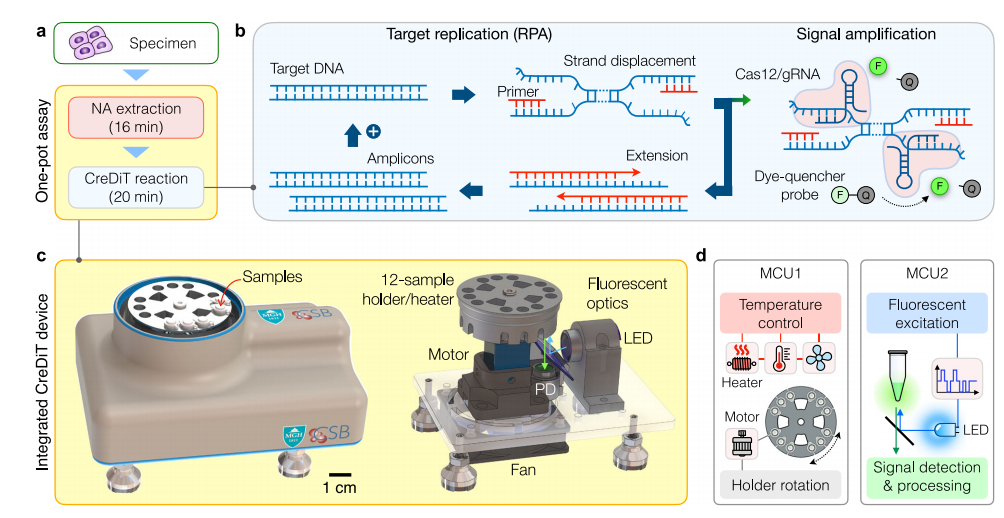
Fig.1 CreDiT assay for HPV detection
II. Robust optical detection for POC operation
Ideal point-of-care (POC) devices aim to reduce design complexity and costs by using fewer components, but this often compromises system reliability and analytical performance, particularly in fluorescent devices. Compared to simple optical setups, the CreDiT optical system using Walsh–Hadamard transform (WHT), inherently compatible with digital electronics and uses a fast, lightweight algorithm (FWHT), requiring only real-number additions and subtractions. The WHT outperformed constant illumination and lock-in techniques, offering superior sensitivity and a detection limit significantly lower (about 2200-fold) than traditional methods, while showing strong correlation with commercial plate reader results.
III. Temperature control for CreDiT reaction
To ensure consistent CreDiT reactions, precise temperature control was incorporated into the assay device. The researchers optimized the sample holder design to facilitate rapid and even heating for all 12 samples.
Moreover, an aluminum holder was designed to ensure the device heated up more rapidly than a solid one. The researchers also tailored the temperature settings to suit different assay steps, ensuring accurate temperature control throughout the process. The uniformity of temperature across all 12 samples was verified by the consistent heat distribution observed during the assay.
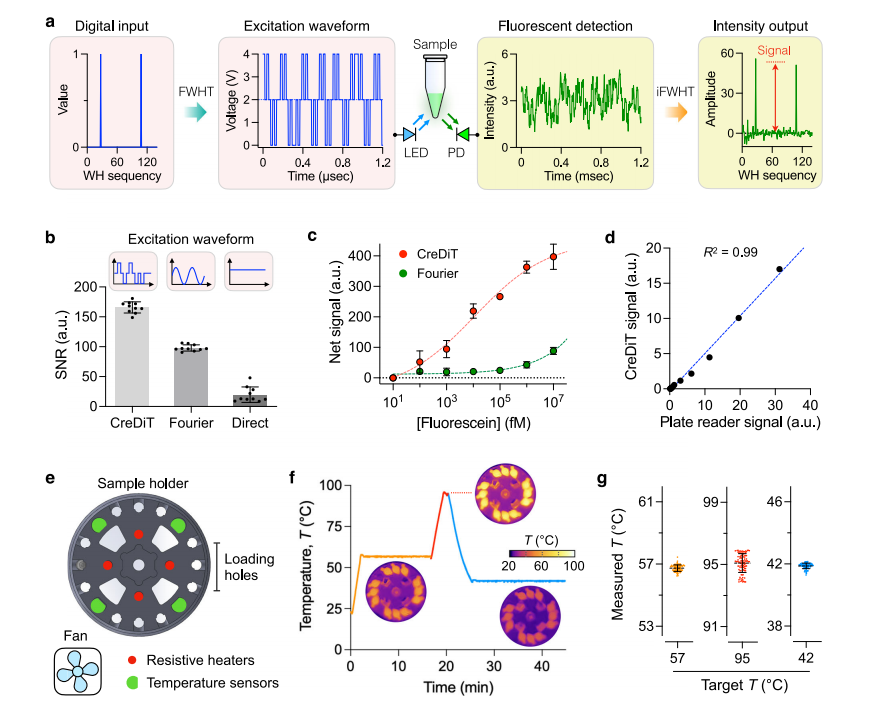
Fig.2 CreDiT system engineering
IV. Assay optimization for POC operation
To optimize the CreDiT assay workflow, the researchers optimized the (NA) extraction process using enzymatic cell digestion, a method that eliminates the need for external instruments and is controlled controlled by simple temperature adjustments. The optimized protocol was compared to a standard extraction method, with NA from both approaches analyzed via PCR and CreDiT assays. The results found that both methods produced NA of similar quality, with strong, comparable PCR bands and similar extraction yields.
The researchers also investigated the feasibility of storing samples and reagents under ambient conditions, a critical consideration for deploying CreDiT in low- and middle-income countries (LMICs). Cells stored in the extraction buffer at 4°C or ambient temperatures showed no significant decline in NA yield for at least two weeks.
Additionally, they lyophilized the premixed CreDiT reagents for easier storage and transport, finding that the reagents retained their effectiveness for at least two weeks at ambient temperatures. These findings suggest that CreDiT can be deployed in resource-limited settings without the need for cold chain solutions, enhancing its practicality and usability.
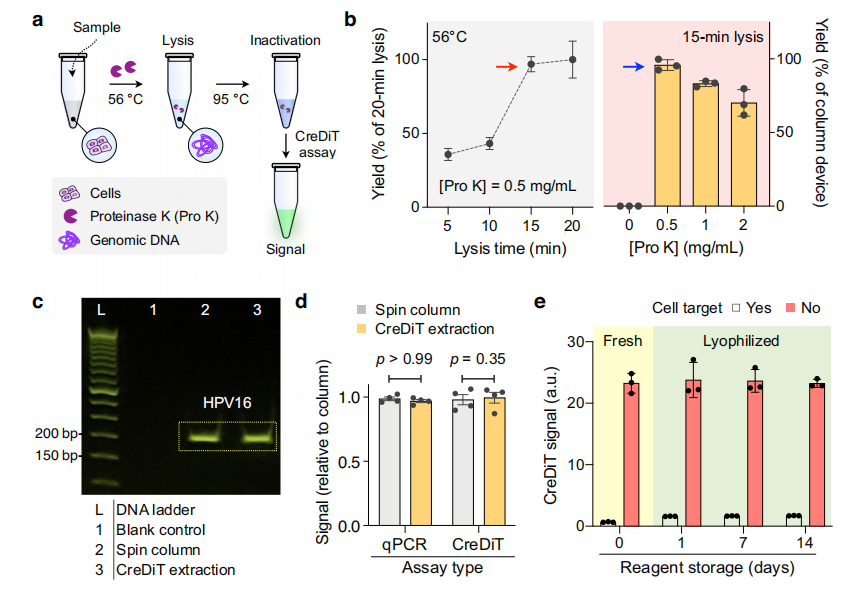
Fig.3 Assay optimization for POC applications
V. CreDiT probe design and validation
A comprehensive library of CreDiT probes was developed for detecting high-risk HPV (hrHPV) by targeting specific DNA sequences. During validation process, the assay reagents were optimized. The results showed that CreDiT signals appeared only when all components , including the target DNA, were present.
Performance comparisons with qPCR and a two-step assay using synthetic HPV16 DNA showed CreDiT had superior sensitivity. CreDiT offered a significantly faster assay time, requiring only 20 minutes, while qPCR took 1 hour and the two-step assay 50 minutes. CreDiT also demonstrated high specificity and minimal cross-reactivity, as validated by both the CreDiT assay and gel electrophoresis, confirming its potential as a robust and sensitive diagnostic tool for hrHPV detection.
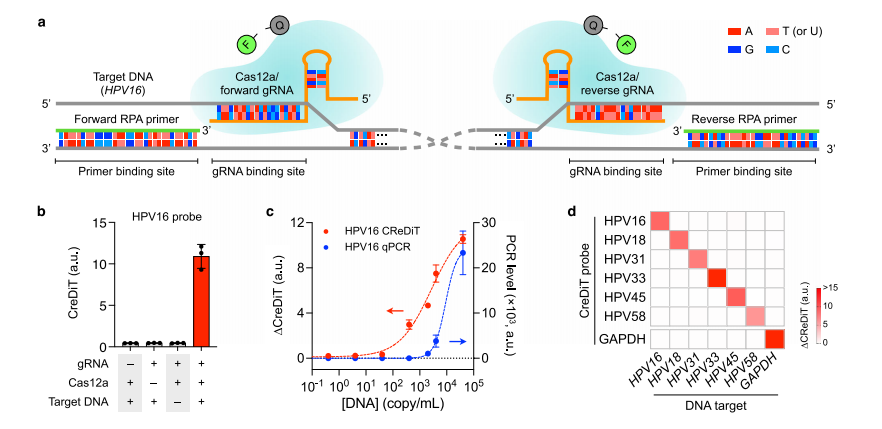
Fig.4 CreDiT probe design
VI. CreDiT assay characterization
To evaluate the consistency of CreDiT with other detection methods, the researchers have characterized the entire CreDiT assay workflow. A panel of cervical cancer cell lines representing different HPV subtypes was used to evaluate additional hrHPV probes. Evaluation with these various hrHPV probes and cervical cancer cell lines confirmed accurate hrHPV genotypes, with only on-target signals detected.
Additionally, CreDiT was adapted for mRNA target detection by incorporating reverse transcriptase, producing results consistent with RT-PCR in both qualitative and quantitative analyses, facilitating the identification of high-risk cases and helping to minimize overtreatment.
VII. CreDiT clinical testing
In the final stage, the CreDiT assay was applied to human clinical samples obtained from cervical or vaginal brushing specimens.
The samples were assessed for HPV status using qPCR method, and residual aliquots were subsequently analyzed using the CreDiT assay. CreDiT accurately distinguished HPV- positive from negative samples and identified three major hrHPV types (HPV16, HPV18, and HPV45), agreeing with clinical diagnostics.
For the three major hrHPV targets, CreDiT showed high diagnostic accuracy. To demonstrate CreDiT's extended clinical utility, anal Pap test samples were also analyzed. Once again, CreDiT results aligned with clinical diagnostic for HPV detection in anal cancer cases. These results highlighted the potential of CreDiT as a reliable and accurate tool for HPV screening in various clinical settings.
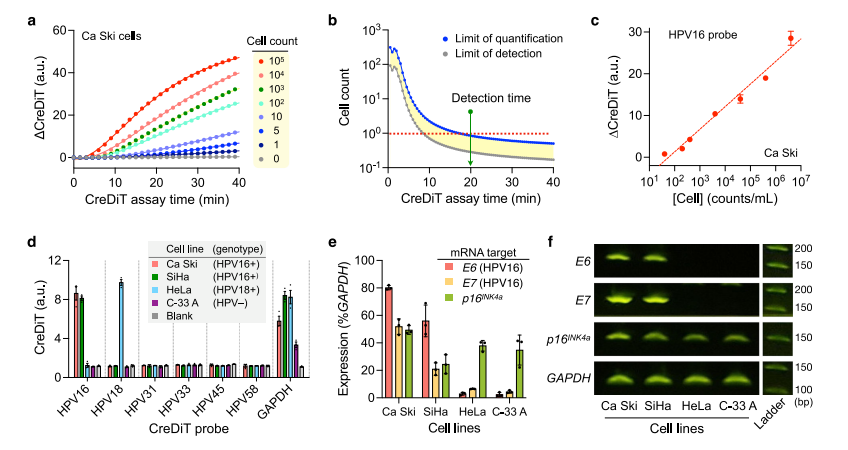
Fig.5 CreDiT assay characterization with cellular samples
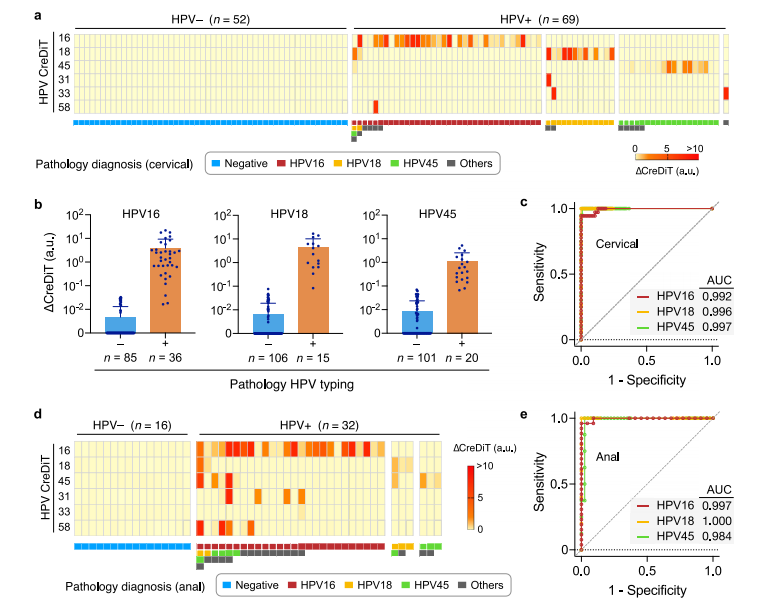
Fig.6 Analysis of clinical samples
In summary, the CRISPR-based CreDiT is designed to offer low-cost, decentralized, automated, and rapid readouts molecular diagnostics for LMICs. Compared to traditional methods, CreDiT enables rapid, same-visit results while significantly reducing costs . However, access to molecular HPV tests in LMICs has been limited due to equipment and cost barriers, resulting in poor clinical outcomes, especially in Africa.
To address these challenges, CreDit was designed with several key features. CreDiT is 100 times more sensitive than PCR, operates in a single-tube format, and maintains a constant temperature of 42°C. The system eliminates the need for PAM sequences, enabling the detection of a broad range of high-risk HPV targets, making it adaptable to various clinical needs. Moreover, the use of a novel digital approach improves the signal-to-noise ratio and computational efficiency, making CreDiT ideal for point-of-care (POC) applications.
However, to further enhance CreDiT’s utility, several challenges remain. In this study, the researchers have proposed expanding the probe library to detect a broader range of HPV subtypes, multiplex detection within a single reaction tube, and streamlining the assay workflow for ease of use. The final goal is to deploy CreDiT in LMICs for real-world assessment, where collaboration with local healthcare providers will help refine the platform, ensuring it meets the specific clinical, economic, and environmental needs of these regions. Ultimately, CreDiT aims to make CRISPR-based detection a practical, field-deployable solution for HPV testing, delivering state-of-the-art screening to areas where it is most needed.
EDITGENE boasts its own independently developed protein purification platform.Our Cas enzymes have demonstrated significantly superior activity compared to other market enzymes through rigorous testing, consistently achieving the highest standards in purity, activity, and sensitivity. Be on the lookout for our isothermal amplification kit and CRISPR detection kits, efficient with minimal equipment requirements and short reaction times—Coming soon!
Recent Blogs:
Follow us on social media
Contact us
+ 833-226-3234 (USA Toll-free)
+1-224-345-1927 (USA)
info@editxor.com










![[Literature Review] A “Gene Scalpel” Emerges: A New Hope for Tay-Sachs Disease Cure](/uploads/20250527/bL2GJjteMDvzmZys_53c82bdd67704fe0e159246934f924ee.png)

Comment (4)