[Literature Review] The Secret Behind Bone “Hollowing”? STOM Protein's Unexpected Function Uncovered
gene knockout

With the global population aging at an accelerating pace, the prevalence of osteoporosis continues to rise, posing a serious public health challenge. Recently, a Chinese research team published a study in Nature Communications, entitled “Targeting lipid raft-related stomatin to ameliorate osteoporosis in preclinical models.”
Using gene knockout technology , the researchers demonstrated that deficiency of the lipid raft-associated protein STOM significantly suppresses osteoclast activity and enhances bone density. This study systematically elucidates the critical role of STOM in the pathogenesis of osteoporosis and reveals its underlying molecular mechanisms. Furthermore, the findings underscore the therapeutic potential of targeting STOM as a novel strategy for osteoporosis treatment.
Original link: https://doi.org/10.1038/s41467-025-60032-9
Spotlight
1. First Identification of the Critical Role of Lipid Raft-Associated Protein STOM in Osteoporosis:
The study revealed that STOM expression is significantly upregulated in bone tissues from both osteoporosis patients and mouse models, suggesting its important role in the pathophysiological regulation of the disease.
2. Elucidation of the Molecular Mechanism by which STOM Regulates Osteoclast Differentiation:
Mechanistically, the researchers found that STOM interacts with the antioxidant protein Prdx1, promoting its degradation via the lysosomal pathway. This process elevates intracellular reactive oxygen species (ROS) levels, thereby enhancing osteoclast differentiation.
3. Validation of Therapeutic Potential Using a CRISPR-Cas9-Based STOM Knockout Model:
Employing CRISPR-Cas9 technology, the team generated STOM knockout mice, and further achieved macrophage-specific knockdown of STOM via AAV delivery. These interventions significantly alleviated ovariectomy (OVX)-induced bone loss, highlighting STOM as a promising therapeutic target for osteoporosis.
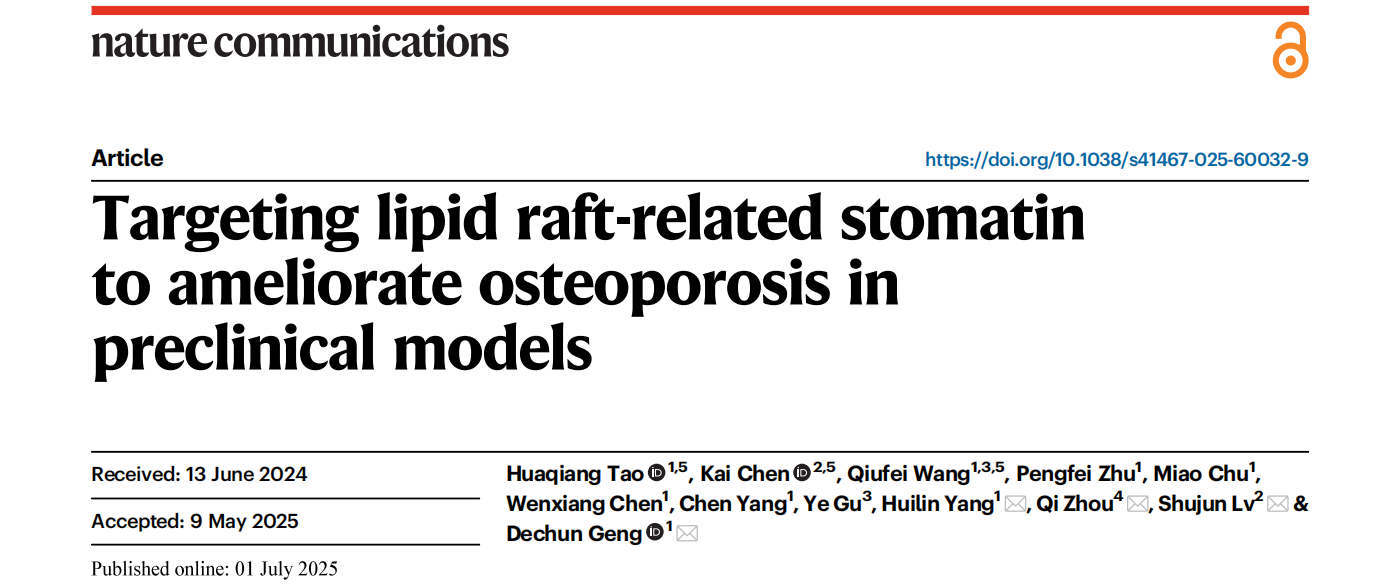
The researchers first analyzed publicly available transcriptomic datasets and found that STOM is highly expressed in both macrophages and mesenchymal stem cells (MSCs). To validate this observation, bone tissue samples were collected from osteoporosis patients and non-osteoporotic controls. Immunofluorescence staining revealed that STOM expression was significantly elevated in both MSCs and macrophages from osteoporosis patients, compared to healthy individuals.
Consistent findings were observed in a classical ovariectomy (OVX)-induced mouse model of postmenopausal osteoporosis. Compared to sham-operated mice, OVX mice exhibited a marked reduction in bone mass along with a notable increase in STOM expression in bone tissues.
The convergence of data from both human samples and animal models provides robust evidence supporting the role of STOM as a key molecule in osteoporosis pathogenesis and a potential diagnostic biomarker.
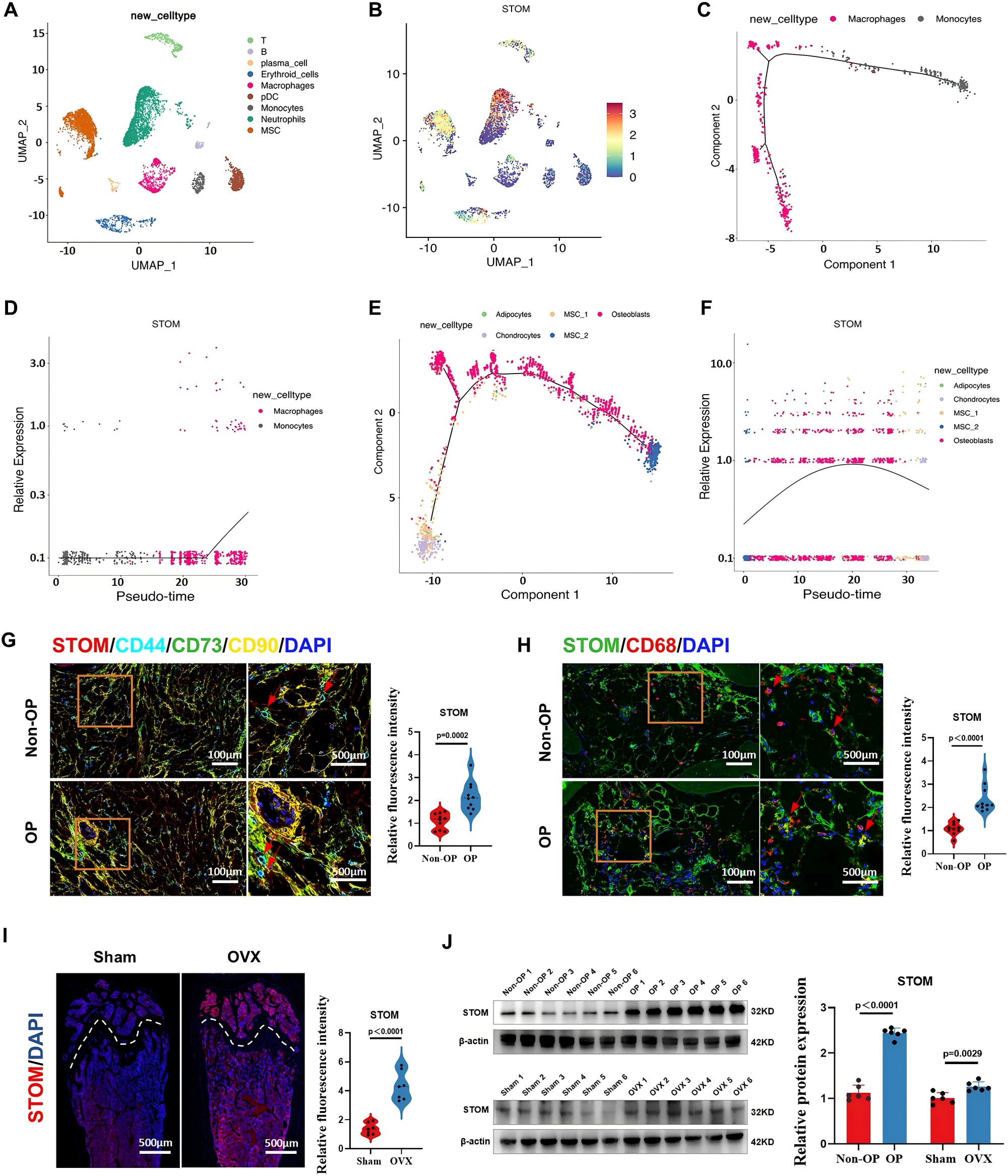
Figure 1. STOM expression in OP patients and OVX mouse models
To further investigate the biological function of STOM, the researchers generated systemic STOM knockout (KO) mice. Interestingly, these mice exhibited a significant increase in bone mass following STOM gene knockout. Subsequent analyses revealed that although STOM knockout led to a mild suppression of osteoblast activity, it had a more pronounced inhibitory effect on osteoclast function, thereby tipping the balance toward bone formation and resulting in increased bone mass. These findings suggest that STOM primarily regulates bone homeostasis by promoting osteoclast activation.
To validate this conclusion, the researchers developed osteoclast-specific STOM knockout mice. Similar to the systemic KO model, these mice showed enhanced bone mass and suppressed osteoclast activity, and were also resistant to ovariectomy (OVX)-induced bone loss. Collectively, these results demonstrate that STOM is a key regulatory factor in osteoclast-mediated bone resorption.
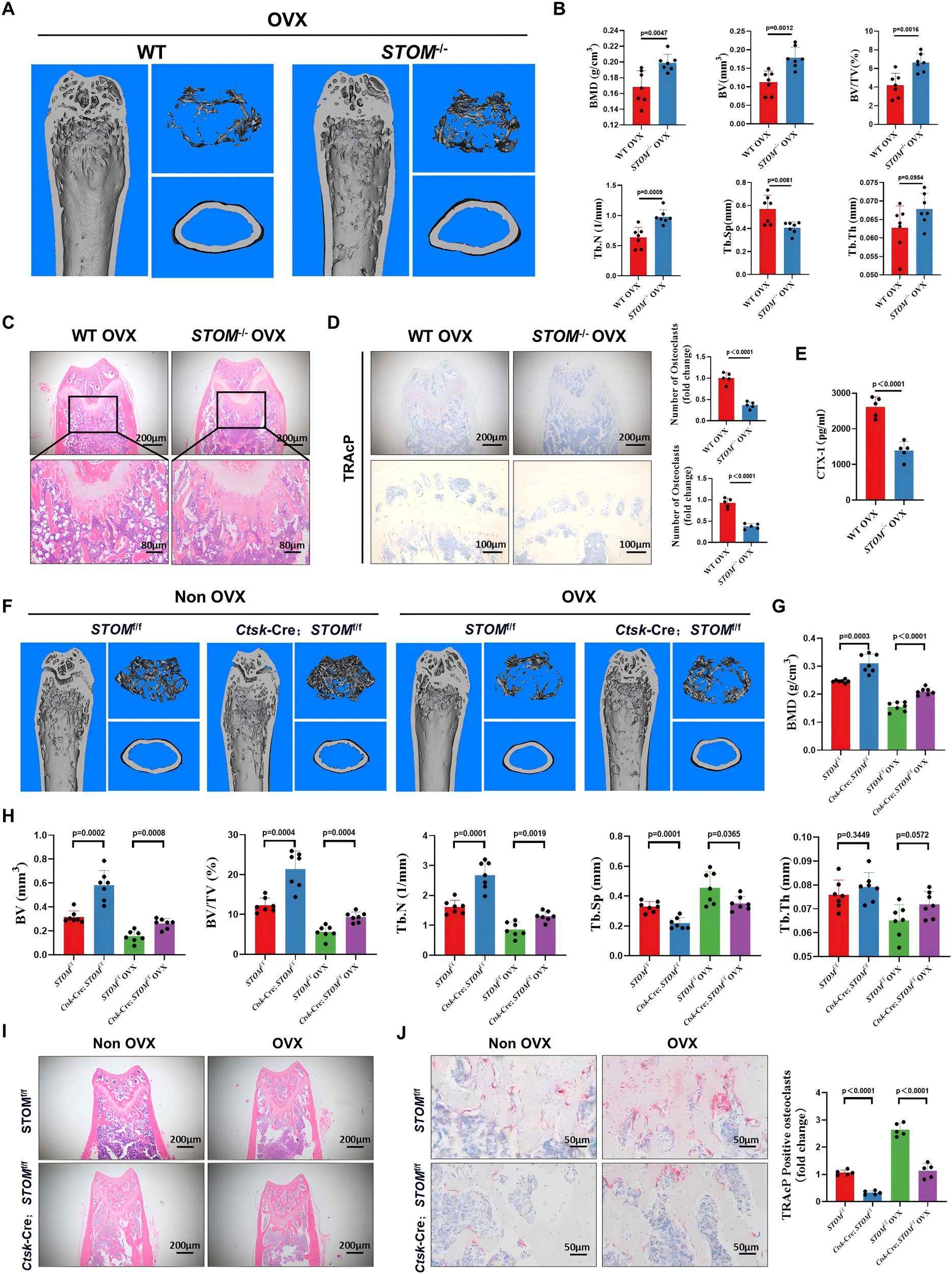
Figure 2. Phenotypic outcomes of systemic and osteoclast-specific STOM knockout
To elucidate the molecular mechanism by which STOM regulates osteoclastogenesis, the researchers performed high-throughput RNA sequencing on bone marrow-derived macrophages from STOM knockout mice. The results showed that STOM deletion led to downregulation of multiple genes involved in osteoclast differentiation, while key genes in the antioxidant pathway, such as Sod1 and Sod2, were significantly upregulated.
Reactive oxygen species (ROS) are metabolic byproducts that, when accumulated excessively, can drive aberrant osteoclast activation. Experimental results confirmed that either STOM knockout or siRNA-mediated STOM knockdown significantly reduced intracellular ROS levels, thereby suppressing osteoclast formation.
Further investigations revealed the underlying mechanism by which STOM regulates ROS homeostasis: through direct interaction with the antioxidant protein Prdx1, STOM facilitates its lysosome-mediated degradation. This degradation diminishes the cellular capacity to neutralize ROS, leading to elevated ROS accumulation and activation of osteoclastogenic signaling pathways.
The identification of this STOM–Prdx1–ROS regulatory axis provides a clear mechanistic explanation for how STOM promotes osteoclast differentiation and drives bone resorption.
Figure 3. STOM-mediated degradation of Prdx1 regulates oxidative stress and osteoclast differentiation
Building upon these mechanistic insights, the researchers engineered an adeno-associated virus (AAV) vector specifically designed to target macrophages and deliver short hairpin RNA (shRNA) for selective knockdown of STOM expression in these cells.
In an OVX-induced mouse model of osteoporosis, the AAV was administered via intra-bone marrow injection. After 8 weeks of treatment, micro-computed tomography (Micro-CT) analysis revealed marked attenuation of bone loss and significant improvement in trabecular architecture in the treatment group.
Histological assessment further showed a substantial reduction in the number of osteoclasts on bone surfaces in mice receiving AAV-STOM shRNA treatment.
These findings demonstrate that targeted inhibition of STOM in macrophages can effectively and safely mitigate bone resorption, highlighting the therapeutic potential of STOM as a clinically translatable target for osteoporosis treatment.
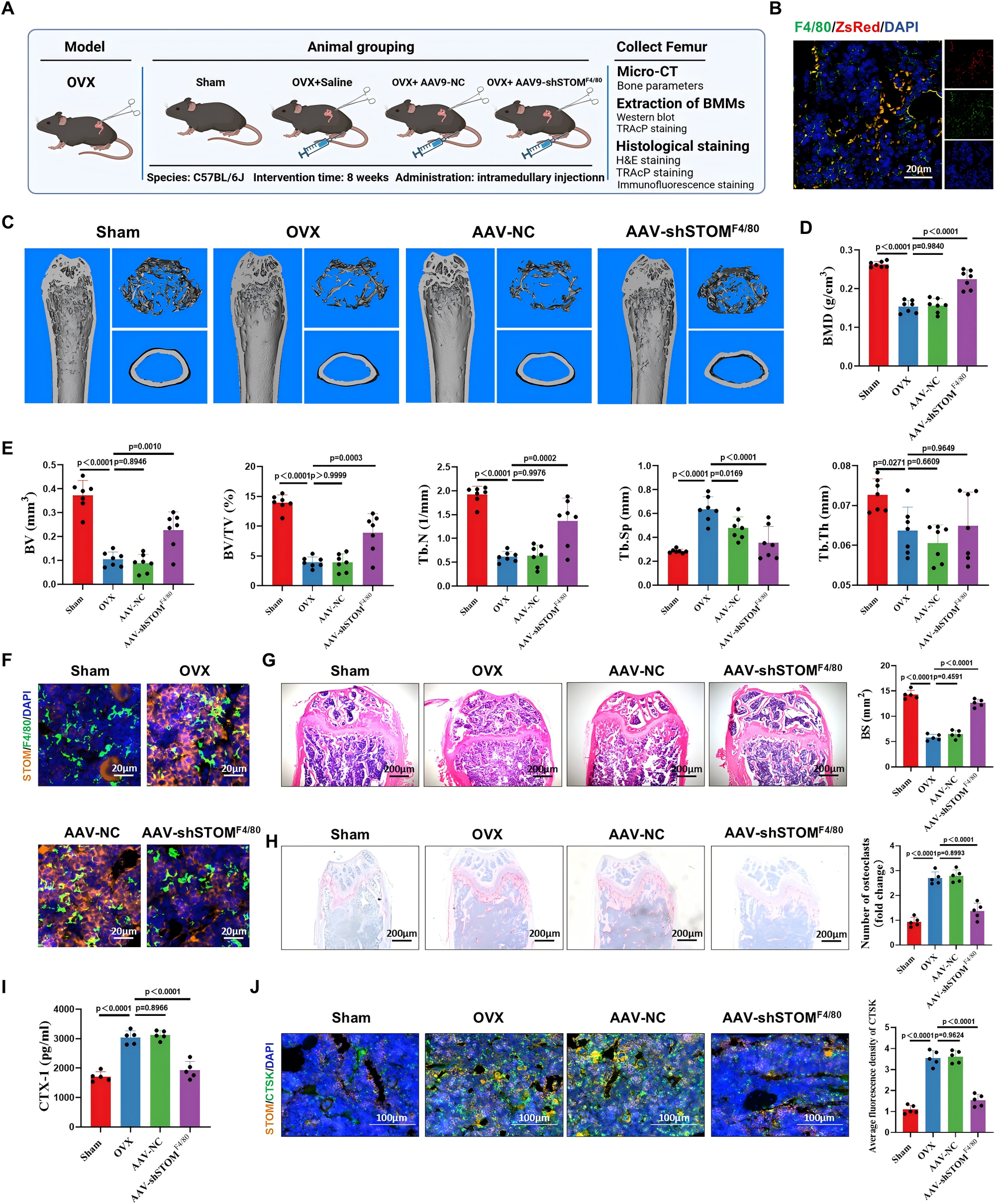
Figure 4. Macrophage-specific STOM knockdown alleviates OVX-induced bone loss
This study systematically reveals the critical role of the lipid raft-associated protein STOM as a positive regulator of osteoclast function in the pathogenesis of osteoporosis. The research not only elucidates a novel molecular mechanism by which STOM promotes osteoclast differentiation through regulation of Prdx1 degradation and subsequent elevation of reactive oxygen species (ROS) levels, but also validates STOM as a promising therapeutic target by employing multiple gene knockout models and developing a macrophage-specific AAV-based intervention strategy. These findings highlight the significant clinical potential of targeting STOM for osteoporosis treatment.
Moreover, preliminary evidence suggests that STOM may also play a key role in other osteoclast-related diseases such as rheumatoid arthritis (RA), possibly through its involvement in the regulation of bone marrow adipocyte differentiation. These discoveries pave the way for future research into the complex functional network of STOM within the bone marrow microenvironment.









![[Literature Review] The Secret Behind Bone “Hollowing”? STOM Protein's Unexpected Function Uncovered](/uploads/20250527/bL2GJjteMDvzmZys_53c82bdd67704fe0e159246934f924ee.png)

Comment (4)