[Literature Review] CRISPR gene editing combined with magnetocaloric activation: A new strategy to assist precision medicine in cancer
gene editing

In current cancer treatment, inducing tumor cell to apoptosis is a key strategy, since tumor cells gain uncontrolled proliferation ability by mechanism of inhibiting normal apoptosis. Gene editing technology, especially the CRISPR-Cas9 system, has received widespread attention due to its ability to precisely regulate the cellular functions. However, the precise control of the system in human body still faces challenges. Combined with magnetothermal therapy, its high permeability and low toxicity can be utilized to provide a new cancer treatment method by controlling the thermal effects and synergistically utilizing CRISPR-Cas9 technology. This magnetothermal - gene editing synergistic therapy demonstrates the promising therapeutic potential and provides a new strategy for precision medicine in cancer.
Recently, a research paper entitled Magnetothermal activated gene editing strategy for enhanced turbine cell apoptosis, was published in Journal of Nanobiotechnology (Top of Zone 1, if: 10.6) from Shanghai Institute of Silicate, Chinese Academy of Sciences. This exciting research proposes a new gene editing strategy - magnetothermal activation gene editing technology, developing an innovative magnetothermal activation CRISPR-Cas9 gene editing system that accurately targets the HSP70 and BCL2 genes of tumor cells, significantly enhancing the tumor cell apoptosis and providing the new possibilities for cancer treatment.

The magnetothermal therapy has become an ideal treatment method due to its high permeability and low toxicity, but it is difficult to completely induce tumor cell to apoptosis when used alone. Combining magnetothermal therapy with the CRISPR-Cas9 system can increase the apoptosis rate of tumor cells at moderate temperatures. A magnetothermal nanoparticle platform has been developed, which utilizes the synergistic effect of alternating magnetic field controlled thermal effects and CRISPR-Cas9 system to target HSP70 and BCL2 genes, thereby enhancing the therapeutic efficacy and reducing the damage to normal tissues. This magnetothermal gene editing therapy shows the great potential for application in precision medicine for tumors.
I. Preparation and Characteristics of Nano Gene Editing System MPDH
In order to construct theMPDH nano gene editing system, researchers first synthesized magnetothermal nanoparticles MNPs (ZnCoFe2O4 @ ZnMnFe2O4) through seed-mediated method. These nanoparticles have a size of about 15.3nm and exhibit the excellent monodispersity. Researchers used C18-PEI to modify the surface of nanoparticles to improve the cell uptake efficiency and loading effectiveness of plasmid DNA, resulting in stable dispersion of MP nanoparticles in water and PBS. Fourier transform infrared spectroscopy (FT-IR) and thermogravimetric analysis (TGA) confirmed the success of surface modification, laying the foundation for subsequent biomedical applications. The MPDH nano gene editing system effectively binds to Cas9 through electrostatic adsorption of plasmids and exhibits its stable size and dispersibility.
To verify the magnetothermal properties of MPDH nanoparticles, researchers evaluated them under a safe magnetic field strength of 1.7 mT. The saturation magnetization of MPDH is 75.4 emu/g, significantly higher than traditional magnetic nanoparticles such as iron or iron oxide. Under an alternating magnetic field of 1.35 kA · m, the temperature of MPDH can rapidly increase from 28℃ to 42℃ at a concentration of 4 mg/mL, making it suitable for mild magnetocaloric therapy. Infrared thermography showed the temperature time distribution of MPDH, and the stability and repeatability of its magnetocaloric properties were demonstrated through cyclic experiments and long-term monitoring, with a temperature stability of around 42℃. These results indicate that plasmids can be released through efficient pH and heat responses, providing the ideal properties for tumor targeted gene editing and precision medicine.
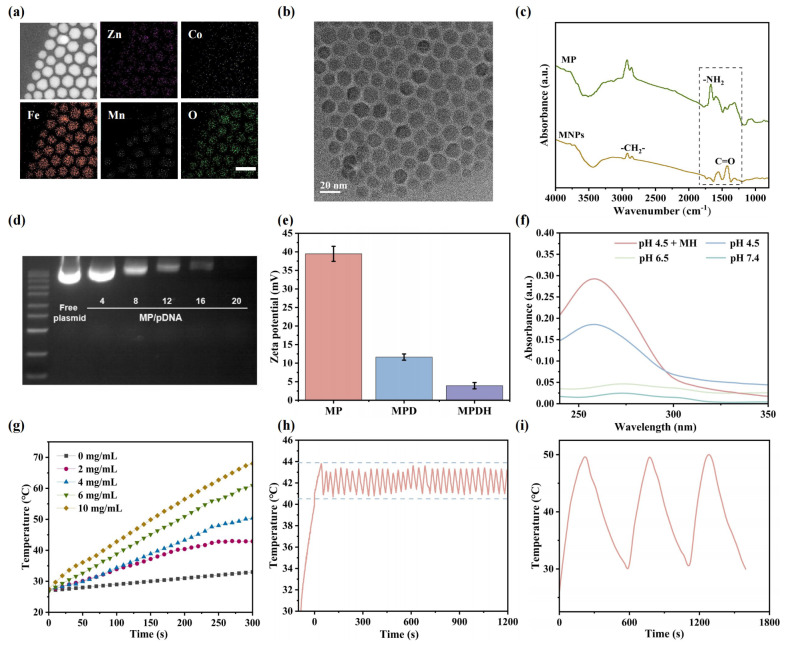
Figure 1: Preparation and characterization of nano gene editing system MPDH
II. Genomic disruption driven by in vitro mild magnetothermal anti-apoptosis
For in vitro treatment studies, researchers first evaluated the cytotoxicity of MP/MPD/MPDH nanoparticles using Cell Counting Kit-8 (CCK-8). The results showed that these nanoparticles had almost no significant toxicity to 4T1 cells at different concentrations, indicating their good biocompatibility. Especially, 4T1 cells treated with MPFD showed the higher cellular activity in the concentration range of 200-800 μg/mL, possibly due to the neutralization of excess positive charges on the surface of polyethyleneimine (PEI) by hyaluronic acid (HA). MPFD nanoparticles also showed the low cytotoxicity towards normal C166 mouse endothelial cells.
Further research has found that the internalization efficiency of MPDH nanoparticles in 4T1 cells is higher than that in C166 cells, indicating that HA modification enhances the targeting of MPDH. Confocal imaging shows that nanoparticles gradually co-localize with lysosomes, and the proton sponge effect of PEI facilitates endosomal escape, thereby promoting plasmid release into the nucleus.
In terms of magnetothermal triggered gene editing, when evaluating transfection efficiency using EGFP plasmids driven by HSP70 promoters, the fluorescence of EGFP in the magnetothermal treated group was significantly stronger than that in the non-magnetothermal induced group. The results of flow cytometry showed that the EGFP positive rate in the mild magnetothermal group was 52.6%, and after 20 minutes of magnetothermal treatment, the proportion of EGFP positive cells reached the highest level (61.4%). This indicates that the magnetothermal controlled CRISPR-Cas9 system can precisely regulate gene editing and reduce non-specific editing events.
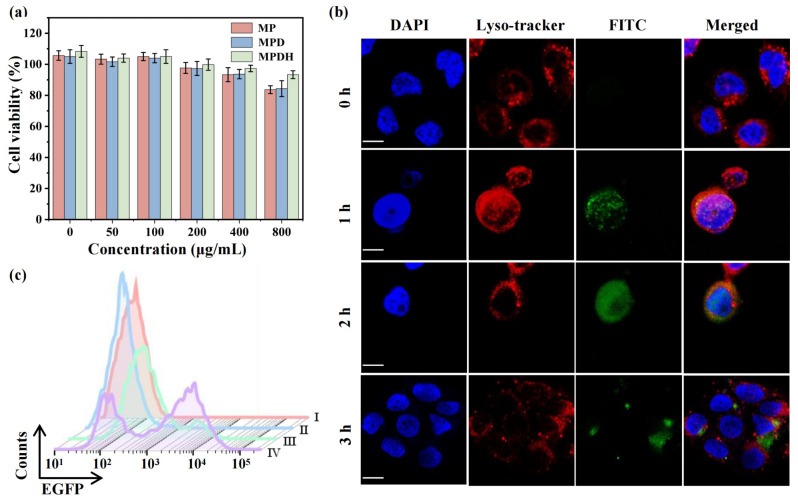
Figure 2: Evaluation of cytotoxicity of MP/MPD/MPDH nanoparticles
III. Exploration of the therapeutic potential of mild magnetic thermal combined with gene editing
In the gene editing of 4T1 tumor cells, the combination of mild magnetothermal treatment significantly enhanced the gene editing effect and promoted tumor cell apoptosis. The apoptosis rate of cells in the magnetothermal treatment group (MH) and the MPDH combined magnetothermal treatment group (MPDH+MH) was significantly higher than that in the control group, with the apoptosis rate in the MPDH+MH group reaching 95.8%. In addition, the CCK-8 assay results showed that the MPDH+MH group significantly inhibited tumor cell proliferation, demonstrating its good therapeutic potential. These results indicate that magnetothermal therapy by targeting tumor apoptosis related genes can effectively upregulate apoptosis and downregulate proliferation in tumor cell.
Researchers analyzed the role of MPDH+MH in inducing cell apoptosis through Western blot and found that the expression of pro-apoptotic proteins Bax, caspase-3, and cytochrome C was upregulated in the MPDH+MH treatment group, while the expression of anti-apoptotic proteins Bcl-2 and HSP70 was downregulated. The MPDH+MH group showed the highest expression of pro-apoptotic proteins and the lowest expression of anti-apoptotic proteins. The mild magnetothermal and CRISPR-Cas9 gene editing system showed a significant synergistic effect in inducing tumor cell apoptosis, achieved through dual regulation of HSP70 and Bcl-2. The magnetothermal method activated the heat shock response pathway, reduced the expression of HSP70 and Bcl-2, and the CRISPR-Cas9 system further reduced the expression of related genes. This mechanism promotes the release of cytochrome C and activation of the apoptotic signaling pathway. The T7 endonuclease I (T7E1) assay showed that the frequency of genomic mutations for HSP70 and BCL2 in the MPDH+MH combined treatment group was significantly higher than that in the single treatment group. And qRT-PCR results also confirmed that the mRNA expression of HSP70 and BCL2 in the MPDH+MH group was significantly reduced. This indicates that the magnetothermal activated nano gene editing system can efficiently and accurately edit the tumor related genes, providing a potential new strategy for in vitro gene therapy.
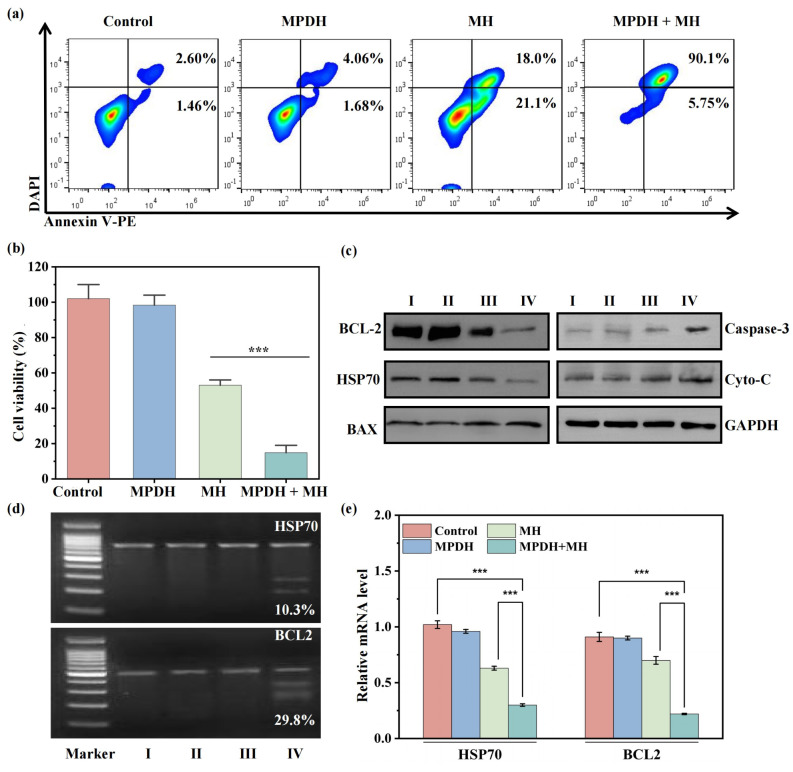
Figure 3: Potential evaluation of magnetothermal activated nano gene editing system
for editing tumor related genes
IV. Magnetothermal mediated gene editing induces tumor apoptosis in vivo
To evaluate the in vivo tumor apoptosis induction and growth inhibition ability of the magnetothermal enhanced CRISPR/Cas9 gene editing system, researchers used a 4T1 tumor-bearing mouse model for assessment. Mice were randomly divided into control group, MPDH treatment group, magnetothermal (MH) group, and MPDH combined with mild magnetothermal (MPDH+MH) group. After injection of MPDH nanoparticles, mice in the MH and MPDH+MH groups received mild magnetothermal treatment for three days. The results showed that the MPDH+MH group significantly inhibited tumor growth, had a high tumor cell mortality rate, significantly reduced tumor size, and achieved a survival rate of 80% on the 35th day of treatment, while the MH group was only 30%. There was no significant difference in weight changes among all treatment groups, indicating a relatively small impact on the overall health of mice.
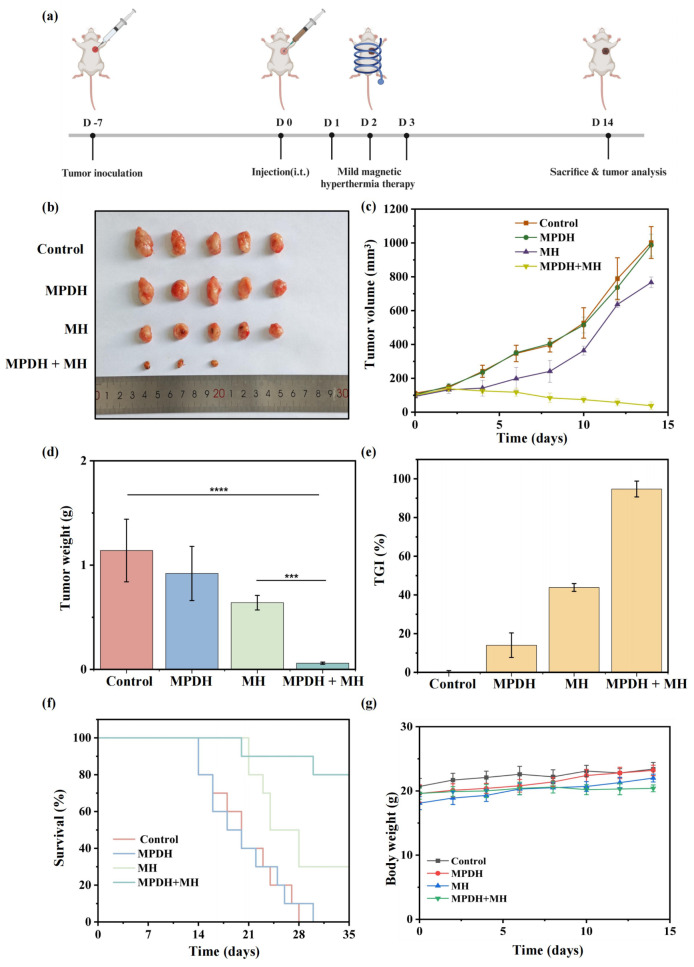
Figure 4: In vivo anti-tumor efficacy of magnetothermal mediated gene editing
To verify the therapeutic effect, researchers performed hematoxylin and eosin (H&E) staining and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining on tumor tissues. As revealed by H&E staining and TUNEL analysis, the MPDH+MH group showed a significant increase in the number of apoptotic and necrotic cells in tumor tissue compared to other treatment groups. The 5Ki-67 antibody staining also confirmed the significant inhibition of tumor cell proliferation activity by the MPDH+MH group, thus validating the significant tumor inhibitory effect of MPDH+MH.
To evaluate the in vivo gene editing effect of MPDH, researchers used Western blot analysis and deep sequencing to assess the expression of HSP70 and BCL2 in tumor tissues. The results showed that the expression levels of HSP70 and BCL2 proteins were significantly reduced in the MPDH+MH treatment group, and mRNA analysis further confirmed the effective knockdown of the genes. In addition, through DNA sequencing, the mutation frequencies of HSP70 and BCL2 genes in the MPDH+MH group were 7.6% and 27.8%, respectively. After treatment, complete blood cell count, serum biochemical analysis, and histopathological examination of organs were performed in mice, and no obvious organ damage or inflammation was found. Blood and liver and kidney function indicators were normal. These results indicate that the gene editing therapy system combining MPDH with magnetothermal therapy has good biosafety during the treatment process.
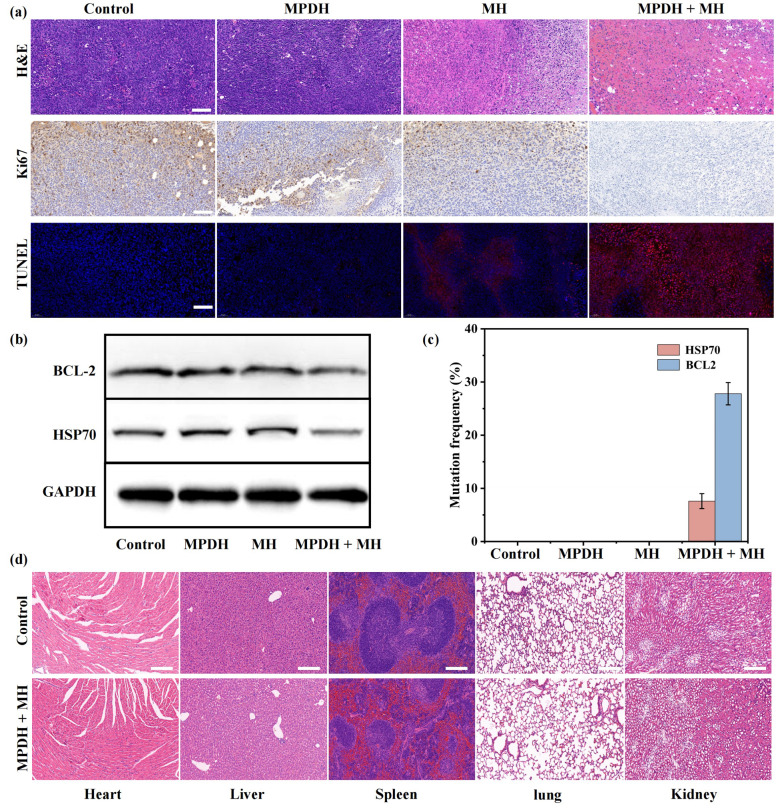 Figure 5: Biosafety Assessment of Magnetothermal Combined Gene Editing Therapy System
Figure 5: Biosafety Assessment of Magnetothermal Combined Gene Editing Therapy System
In summary, this study innovatively designed a thermal-responsive nanosystem that can utilize the magnetothermal effect to facilitate precise gene editing of the CRISPR-Cas9 system in tumor cells. Through mild heating induced by an external magnetic field, the system can regulate the expression of key proteins such as HSP70 and BCL2, thereby achieving targeted killing of cancer cells. This method effectively improves the efficiency and specificity of tumor treatment while protecting the surrounding healthy tissues. The progress of CRISPR-Cas9 technology provides a new precision medicine strategy for tumor treatment and offers potential for clinical applications.
Original Article Link:https://doi.org/ 10.1186/s12951-024-02734-8
EDITGENE focuses on CRISPR technology, offering a range of high-quality gene editing services and in vitro diagnostic products. These include but are not limited to: CRISPR Library Screening, Cell Line Engineering, Monoclonal Cell Line Screening, CRISPR Detection, we are committed to providing the most efficient technical services for CRISPR-related, gene functional research, in vitro diagnostics, and therapeutic research.
Recent Blogs
- 1. [Weekly News] Multiplexed CRISPR Arrayed Libraries: Advancing Whole-Genome Targeting for Knockout, Activation, and Silencing Research
- 2. [Weekly News] CRISPR Library Screening: The Key Role of EV-DNA Structure in Kupffer Cell Mediated Antitumor Immunity
3. [Star of the Month] Human Whole Genome CRISPR/Cas9 Knockout Library and Human RNA Binding Protein Library
Follow us on social media
Contact us
+ 833-226-3234 (USA Toll-free)
+1-224-345-1927 (USA)
info@editxor.com













![[Literature Review] CRISPR gene editing combined with magnetocaloric activation: A new strategy to assist precision medicine in cancer](/uploads/20241216/zbPkGXgmUTx2soEf_53c82bdd67704fe0e159246934f924ee.png)

Comment (4)