Cracking the Delivery Puzzle: How Can Gene Editing Precisely Reach Target Cells?
CRISPR/Cas9

The CRISPR/Cas gene editing system, often referred to as "genetic scissors", has found widespread applications in life sciences, agriculture, and the food industry. But how can these "scissors" be efficiently delivered to target cells to carry out precise editing? This article introduces the commonly used delivery methods, vectors, and their applications in gene editing.
I. Delivery Methods for the CRISPR/Cas9 System
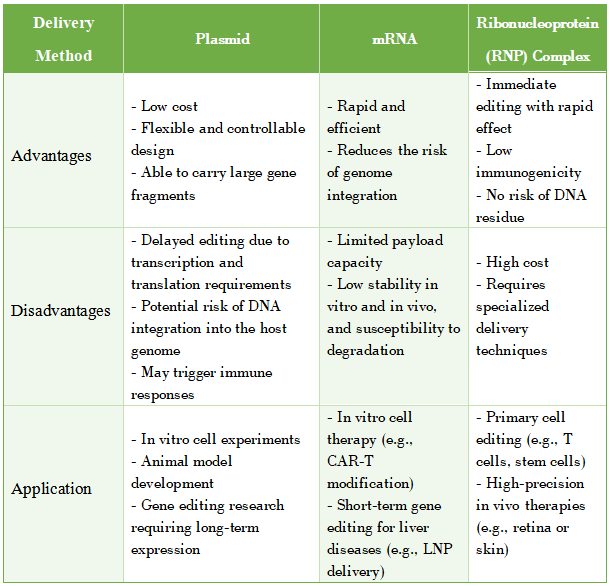
(A). Plasmid-Based Delivery of Cas9 Protein and sgRNA
Advantages: Cost-effective, highly flexible in design, and capable of carrying large gene fragments.
Disadvantages: Before gene editing can take place, plasmids must undergo transcription and translation in the cytoplasm, which makes the process slower. Additionally, plasmid delivery carries the risk of DNA integration into the host genome and may trigger immune responses.
(B). mRNA-Based Delivery of Cas9 Protein and sgRNA
Advantages: Rapid and efficient, with a lower risk of genomic integration.
Disadvantages: Limited payload capacity, low stability in vitro and in vivo, and susceptibility to degradation, which can impact delivery efficiency.
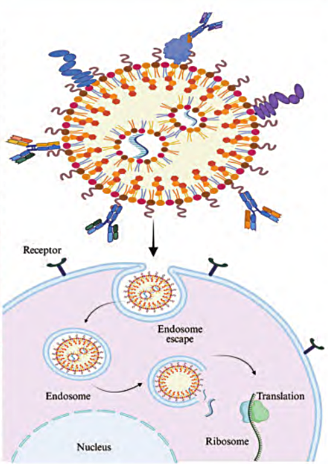
(C). Direct Delivery of Cas9 Protein and sgRNA as a Ribonucleoprotein (RNP) Complex (~10 nm in size)
Advantages: Enables immediate gene editing, has low immunogenicity, and eliminates the risk of genome integration.
Disadvantages: Currently, this method is costly and has low delivery efficiency in vivo.
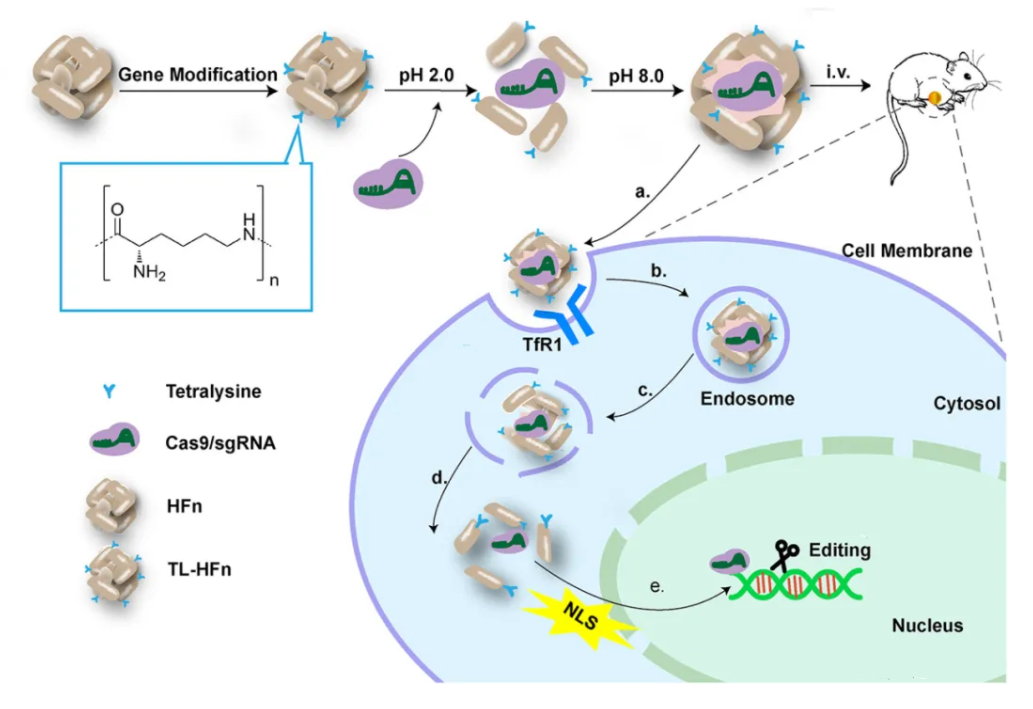
II. Delivery Vectors
Gene-editing system delivery vectors can be generally categorized into viral and non-viral vectors. Commonly used viral vectors include lentiviruses, adenoviruses, and adeno-associated viruses (AAV). Non-viral methods mainly involve nanoparticle-based delivery systems and physical delivery methods.
(A) Viral Vector Delivery
Mechanism: Viral vector-based delivery utilizes the characters of viruses to infect cells and release genetic material. The most commonly used viral vectors include:
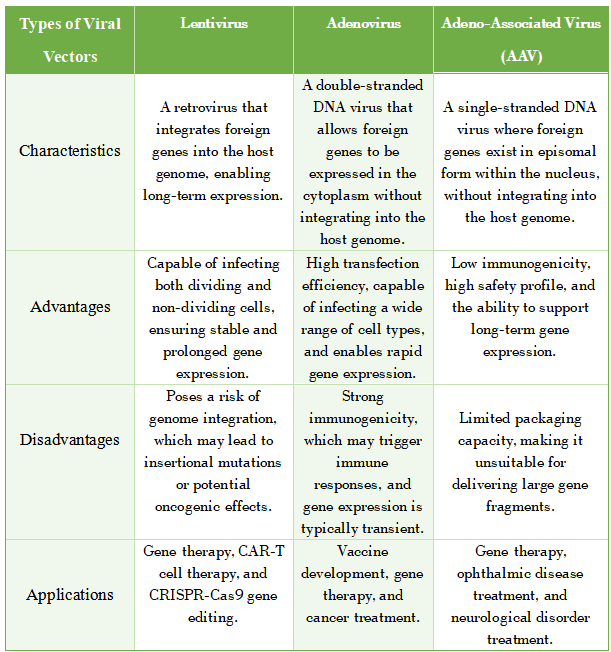
1. Lentivirus
Characteristics: Lentiviruses is a type of retrovirus capable of integrating foreign genes into the host genome, allowing for long-term gene expression.
Advantages: Capable of infecting both dividing and non-dividing cells, ensuring stable and prolonged gene expression.
Disadvantages: Poses a risk of genome integration, which may lead to insertional mutations or potential oncogenic effects.
Applications: Commonly used in gene therapy, CAR-T cell therapy, and gene editing technologies such as CRISPR-Cas9.
2. Adenovirus
Characteristics: Adenovirus is double-stranded DNA virus that can attach and enter the cell. Once inside, the exogenous gene is expressed in the cytoplasm without integrating into the host genome.
Advantages: High transfection efficiency, capable of infecting a wide range of cell types, and enables rapid gene expression.
Disadvantages: Strong immunogenicity, which may trigger immune responses, and gene expression is typically transient.
Applications: Commonly used in vaccine development, gene therapy, and cancer treatment.
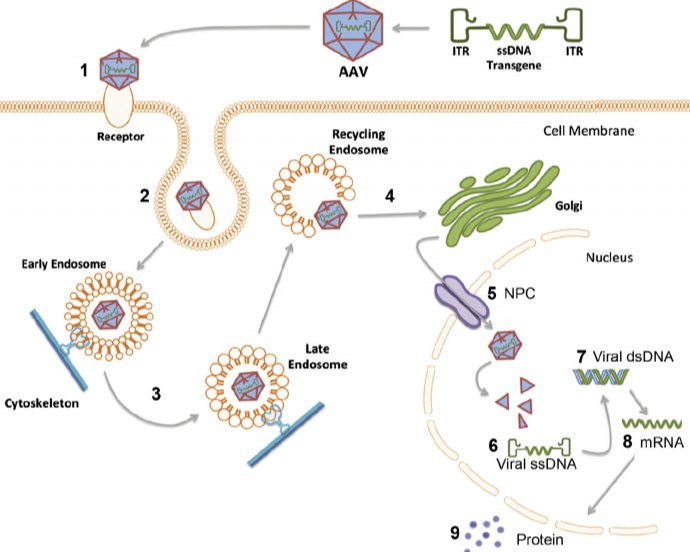
3. Adeno-Associated Virus (AAV)
Characteristics: AAV is a single-stranded DNA virus that delivers foreign genes as episomes in the nucleus without genome integration.
Advantages: Low immunogenicity, high safety profile, and the ability to support long-term gene expression.
Disadvantages: Limited packaging capacity, making it unsuitable for delivering large gene fragments.
Applications: Commonly used in gene therapy, ophthalmic disease treatment, and neurological disorder treatment.
(B) Nanoparticle-Based Delivery Systems
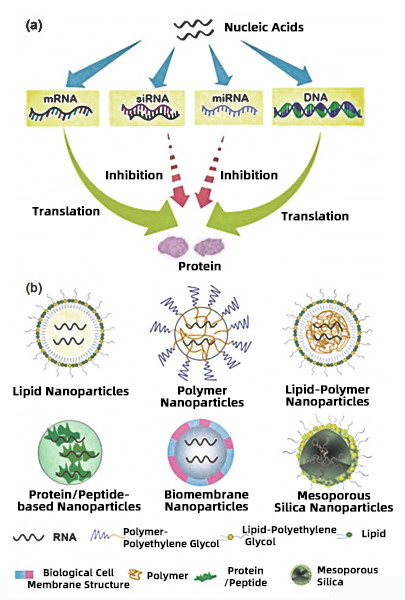
Mechanism: Nanoparticle-based delivery system utilizes positively charged nanoscale carriers to encapsulate negatively charged gene-editing systems. These carriers transport the gene-editing components to target cells or tissues, where they enter cells through endocytosis or lipid membrane fusion. Common types of nanoparticles include:
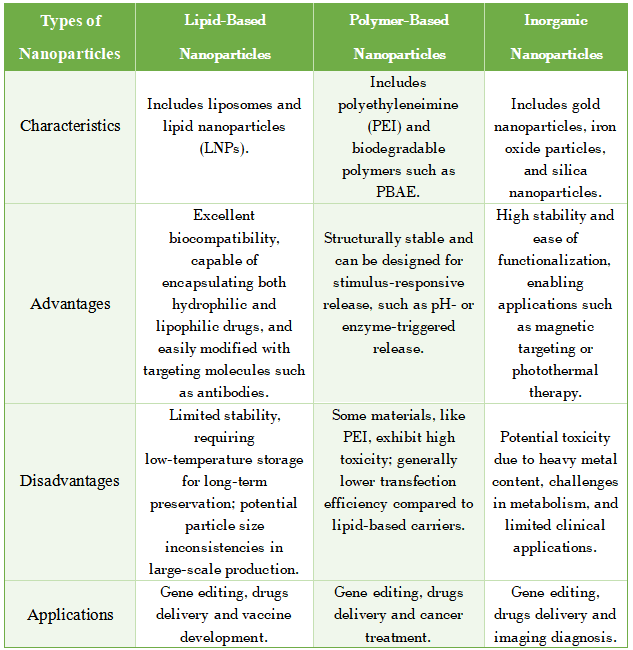
1. Lipid-Based Nanoparticles
This category includes liposomes and lipid nanoparticles (LNPs).
Advantages: Excellent biocompatibility, capable of encapsulating both hydrophilic and lipophilic drugs, and easily modified with targeting molecules such as antibodies.
Disadvantages: Limited stability, requiring low-temperature storage for long-term preservation; potential particle size inconsistencies in large-scale production.
2. Polymer-Based Nanoparticles
Includes polyethyleneimine (PEI) and biodegradable polymers such as PBAE.
Advantages: Structurally stable and can be designed for stimulus-responsive release, such as pH- or enzyme-triggered release.
Disadvantages: Some materials, like PEI, exhibit high toxicity; generally lower transfection efficiency compared to lipid-based carriers.
3. Inorganic Nanoparticles
This category includes gold nanoparticles, iron oxide particles, and silica nanoparticles.
Advantages: High stability and ease of functionalization, enabling applications such as magnetic targeting or photothermal therapy.
Disadvantages: Potential toxicity due to heavy metal content, challenges in metabolism, and limited clinical applications.
(C) Physical Delivery Methods
Commonly used physical delivery methods include electroporation and microinjection.
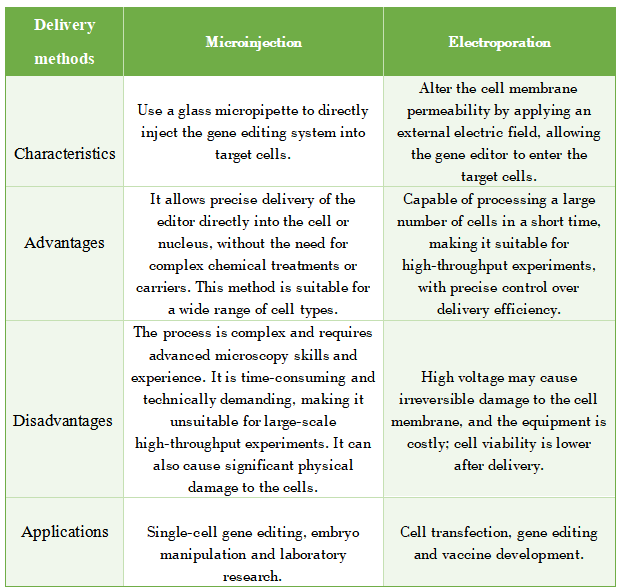
1. Microinjection
This method uses a glass micropipette to directly inject the gene editing system into target cells.
Advantages: It allows precise delivery of the editor directly into the cell or nucleus, without the need for complex chemical treatments or carriers. This method is suitable for a wide range of cell types.
Disadvantages: The process is complex and requires advanced microscopy skills and experience. It is time-consuming and technically demanding, making it unsuitable for large-scale high-throughput experiments. It can also cause significant physical damage to the cells, affecting cell survival and subsequent function.
2. Electroporation
In this method, the target cells are placed in an external electric field, which alters the cell membrane's permeability, allowing the gene editor to enter the cells.
Advantages: It can process a large number of cells in a short time, making it suitable for high-throughput experiments. By adjusting parameters like electric field strength, pulse duration, and waveform, the delivery efficiency can be precisely controlled.
Disadvantages: High voltage may cause irreversible damage to the cell membrane, potentially leading to cell death. The equipment is expensive, and the cell viability after delivery tends to be lower.
This article offers a comprehensive overview of gene editing delivery methods, aiming to provide a better understanding of the applications and challenges associated with gene editing technology.
EDITGENE focuses on CRISPR technology, offering a range of high-quality gene editing services and in vitro diagnostic products. These include but are not limited to: CRISPR Library Screening, Cell Line Engineering, Monoclonal Cell Line Screening, CRISPR Detection, We are committed to providing the most efficient technical services for CRISPR-related, gene function research, in vitro diagnostics, and therapeutic research.
Recent Blogs
- 1. [Literature Review] CRISPR–Csm: Real-time Single-Molecule Tracking of RNA Dynamics in Live Cells
- 2. [Literature Review] New Developments in Prime Editing: Phage-Assisted Evolution and Protein Engineering Yield More Efficient and Compact Prime Editors
- 3. [Literature Review] A New Hope for Progeria Treatment: CRISPR Gene Editing Uncovers Protein Synthesis Dysregulation and Novel Therapeutic Targets
Follow us on social media
Contact us
+ 833-226-3234 (USA Toll-free)
+1-224-345-1927 (USA)
info@editxor.com















Comment (4)