【New Trends in Gene Editing】CTLA4, MUC1, and mTOR Knockout Cells
Gene knockout cells refer to the use of CRISPR technology for gene editing. By designing specific sgRNA targeted to the cleavage site of the target gene, the Cas9 protein combines with sgRNA to “cut out” the desired gene from the cell, thereby achieving gene knockout. Gene knockout cells have a wide range of applications in life sciences, medicine, and pharmaceutical research, such as establishing disease models, drug screening and target validation, and gene therapy by knocking out pathogenic genes. This article selects three related studies on gene knockout cells to walk you through latest progress on this cutting-edge technology.
Knockout of CTLA4 Gene Enhances CAR T-Cell Efficacy, Offering a New Strategy for Cancer Treatment
Original link: https://doi.org/10.1016/j.immuni.2023.09.001
Chimeric Antigen Receptor T-cell (CAR T-cell) therapy has made significant progress in the treatment of B-cell malignancies. Although this therapy has been successful in some patients, researchers have noticed that not all patients can produce lasting responses. Researchers have attempted to understand the mechanisms behind the failure of CAR T-cell therapy and explored the possibility of restoring CAR T-cell function by knocking out CTLA4.
Researchers used CRISPR-Cas9 technology to knock out CTLA4 gene in CAR T-cells, creating CTLA4 knockout CAR T-cells (CART19 cells), which express CD19-specific chimeric antigen receptors. CART19 cells showed enhanced anti-tumor activity and proliferative capacity in vitro, and demonstrated better function under chronic antigen exposure (CAE) stress tests. In mouse models of leukemia and multiple myeloma xenografts, CART19 cells improved tumor control, increased survival rates, and enhanced T-cell expansion. Knockout of the CTLA4 gene reactivated T-cells in patients with dysfunctional chronic lymphocytic leukemia (CLL). CTLA4 gene knockout cells have enhanced the therapeutic potential of CAR T-cells, helping to maintain CAR expression on the T-cell surface and enhance cellular effector functions, providing a new strategy for CAR T-cell therapy.
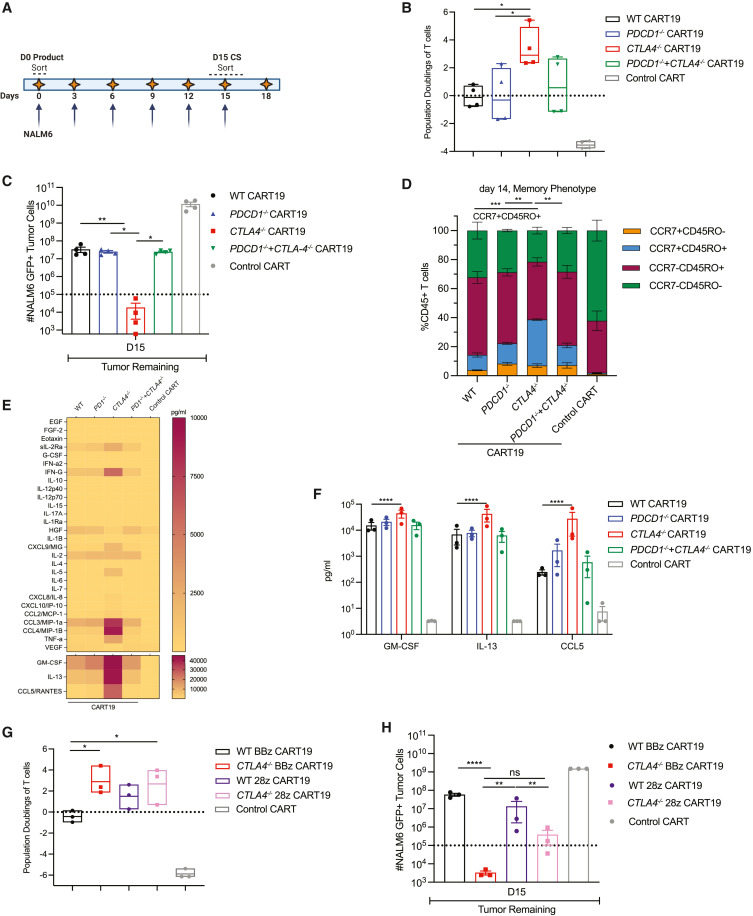
Figure 1: Deletion of the CTLA4 gene confers superior in vitro effector function to CART19 cells under stress conditions.
MUC1 Knockout Cells Aid in the Study of the Pathogenesis of Pancreatic Cancer
Original link: https://doi.org/10.1073/pnas.2315509121
Pancreatic ductal adenocarcinoma is a highly aggressive cancer that is often diagnosed in the late stages, leading to poor patient outcomes. Despite extensive research efforts, the molecular mechanisms underlying pancreatic cancer metastasis remain incompletely understood. Currently, the treatment of pancreatic cancer primarily involves surgical resection following chemotherapy, but the effectiveness of most current standard-of-care therapies is limited due to late diagnosis, early tumor metastasis, and the development of chemotherapy resistance.
Researchers generated MUC1 stably knockout cells in pancreatic cancer cell lines using the CRISPR/Cas9 system to investigate the impact of MUC1 on gene expression in the polyamine metabolism pathway. The study found a positive correlation between MUC1 expression and the expression of the key gene SAT1 in the polyamine metabolism pathway. MUC1 knockout significantly reduced the growth of pancreatic cancer cells in 3D sphere experiments and consistently decreased the expression levels of SAT1 and SMS genes across all pancreatic cancer cell lines. In this study, MUC1 knockout cells revealed the crucial role of the MUC1-HIF-1α-SAT1 signaling axis in pancreatic cancer, providing potential targets for the development of new therapies against pancreatic cancer.
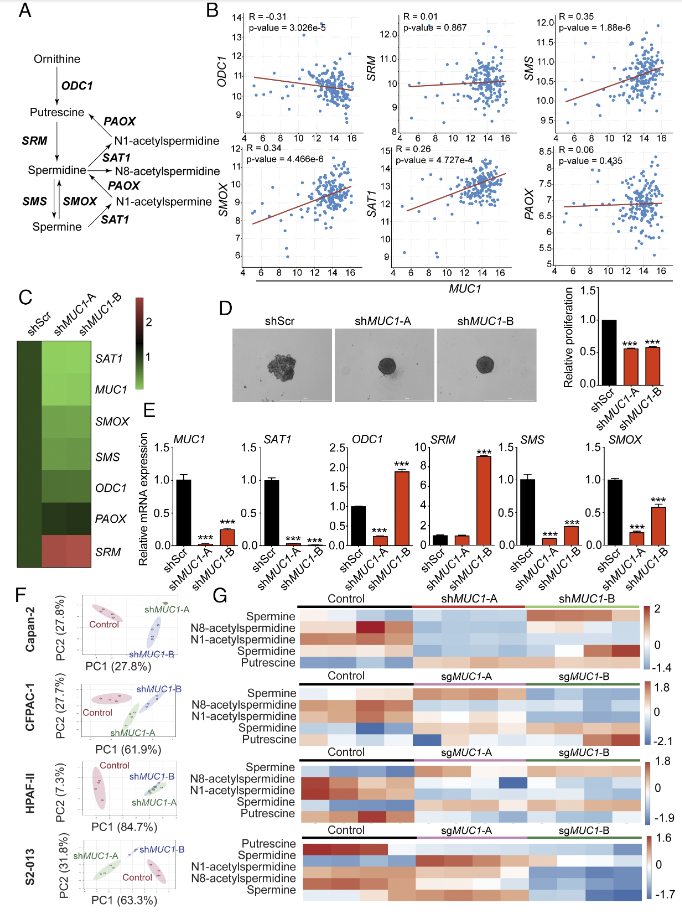
Figure 2: MUC1 is positively correlated with the polyamine metabolic pathway gene SAT1
mTOR Knockout Cells Reveal the Important Role of the mTOR Signaling Pathway in Regulating MR Activity
Original link: https://doi.org/10.1210/endocr/bqae015
The mineralocorticoid receptor (MR) is a ligand-activated transcription factor within the steroid hormone receptor superfamily, capable of mediating a variety of cell-specific functions, including nutritional effects on cells and promoting fluid/electrolyte homeostasis. Inappropriate activation of MR can lead to hypertension, cardiovascular, renal, inflammatory, and metabolic pathologies. Understanding the mechanisms that regulate MR activation is crucial for developing new therapeutic approaches that target inappropriate MR activity while preserving its other essential functions.
Related studies have shown that mTOR regulates MR by affecting the activity of ULK1 (Unc-51 like kinase 1). ULK1 can phosphorylate MR, thereby inhibiting its activation. mTOR prevents the inactivation of MR by phosphorylating and deactivating ULK1. Therefore, researchers used CRISPR/Cas9 technology to knock out specific adaptor protein genes of the mTOR complex 1 (mTORC1) and complex 2 (mTORC2), reducing the level of phosphorylated ULK1, decreasing the activation of ligand-induced MR reporter genes, and the transcription of endogenous MR target genes. The study of mTOR knockout cells revealed two ways in which mTOR regulates MR activity, providing new insights into the role of mTOR in cellular metabolism and growth, and contributing to the development of new therapies for MR-related diseases.
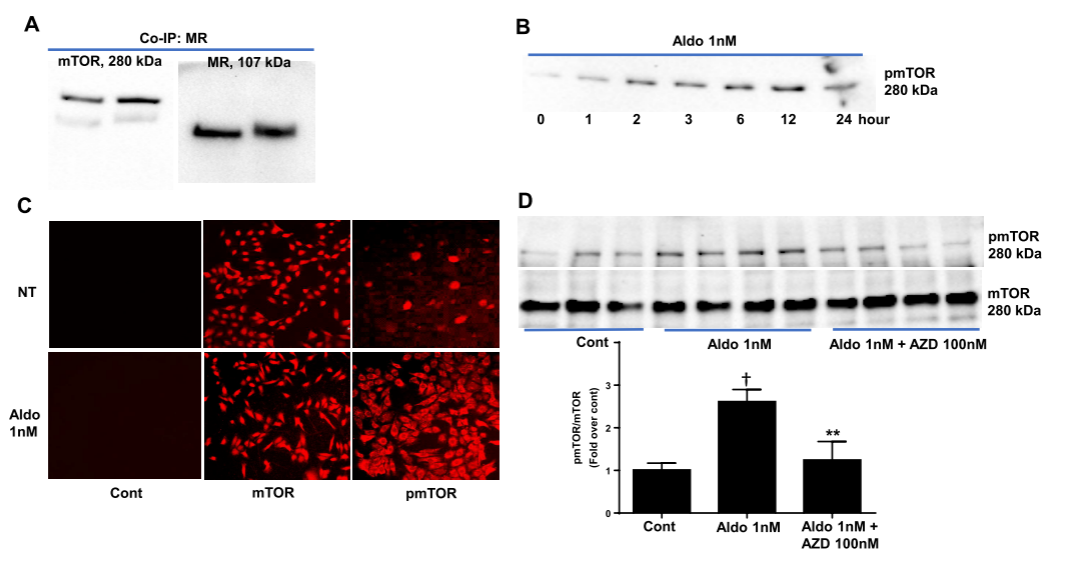
Figure 3: Interaction between Mineralocorticoid Receptor and mTOR
- EDITGENE boasts a ready-to-ship inventory of over 3,800 gene knockout cell lines, covering popular targets such as CTLA4, MUC1, and mTOR mentioned in the above articles. Place your order now and receive delivery within a week. Enjoy our May special offer, starting at just $2230 for KO cells! Click here to view promotion details >>>
You may be interested in:
1. [Mother’s Day bundle Day Bundle | EDITGENE Cell Line Bundle]
2. Pre-experiment essentials: A walkthrough of the CRISPR Assay
3. [Frontier Information] The first AI gene editor has successfully edited the human genome










![[Literature Review] A Novel Mechanism of Cisplatin Resistance in Osteosarcoma: CRISPR Screening Identifies Key Regulators in Organoid Models](/uploads/20250527/bL2GJjteMDvzmZys_53c82bdd67704fe0e159246934f924ee.png)
![[Quality Share] Decoding Point Mutations: A Comprehensive Guide to Three Common Construction Methods](/uploads/20250328/ESzk5OC49wpxIHVv_3cbfa5e98ea1d238127fe23c72b0f4b2.png)

Comment (4)