[Research Frontier] New Trends in Prime Editing: Point Mutated Cells for Preclinical Gene Therapy
Prime editing is an improved gene editing technology based on the CRISPR/Cas system to achieve precise fragment insertion, deletion and arbitrary substitution of bases at the target site of the genome, with higher accuracy and lower error rate.
When it came out, it attracted widespread attention from many scientific researchers, and extensive research was conducted on the treatment of hereditary diseases, gene mutation diseases and cancers. For example, by correcting the mutant genes to treat genetic diseases, or by editing oncogenes in cancer cell genomes to prevent them from growing and spreading. Following three articles are about the clinical treatment of Prime Editing, bringing you the latest research trends.
Prime editing helps in the treatment of β - thalassemia, and no off-target effect was detected .
β - thalassemia is a hematopoietic system disease caused by a single gene mutation in the hemoglobin (HGB) subunit beta gene (HBB), resulting in abnormal expression of beta-globin. To date, more than 300 β-thalassemia alleles have been discovered, of which intronic point mutations are the most common type, leading to activation of aberrant splicing sites.
In this study, prime editor version 3 (PE3) was used to genetically correct the mouse model of human β-thalassemia IVS-II-654 mutation (C > T ), and the PE3 component was microinjected into IVS-II-654 mice fertilization eggs to produce mutant-editing mice. Genome sequencing results of the IVS-II-654 site showed that the editing efficiency of PE3 was 14.29%; RT-PCR analysis showed that PE3-induced gene editing restored normal splicing of β-globin mRNA; Comprehensive phenotypic analysis of thalassemia symptoms Corrected IVS-II-654 mice were shown to have the same normal phenotype as wild-type mice.
The researchers constructed a plasmid containing Cas9, reporter gene enhanced green fluorescent protein (EGFP) and sgRNA, and transferred two plasmids Peg and Ni targeting different sites into human HEK293T cells. The three off-target sites most likely to generate indels were deeply sequenced for off-target analysis of pegRNA (Peg) and nick sgRNA (Ni). The results showed that the efficiency of indel generation was <0.35%, indicating that the PE3-mediated gene therapy strategy has good safety for β-thalassemia IVS-II-654 mutation.
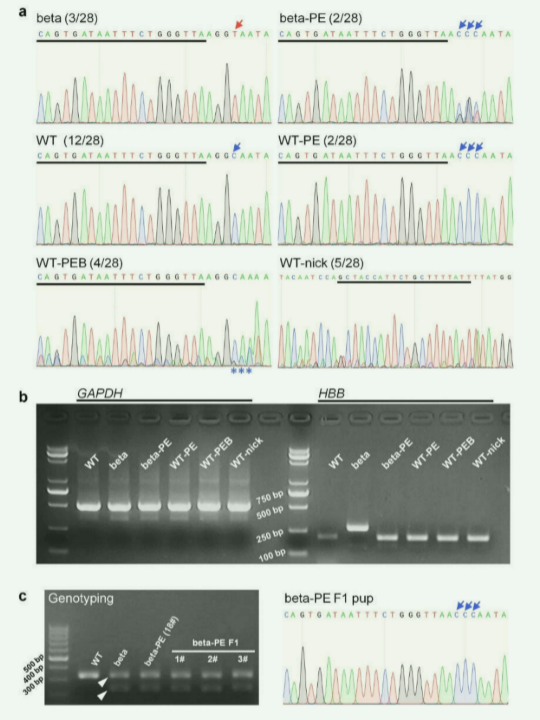
Figure 1 The Prime editing system accurately completes gene editing for the treatment of β-thalassemia
CRISPR-Cas9 knockout of key genes in hPSCs significantly improves the editing efficiency of the PE system
Precise genome editing of human pluripotent stem cells (hPSCs) has potential applications in genetic disease modeling and in vitro stem cell therapy. Unlike fully differentiated somatic cells, hPSCs have unique cellular characteristics that maintain genome integrity, requiring higher accuracy and efficiency from the gene editing tool Prime editing.
Researchers used CRISPR-Cas9 technology to knock out the homozygous genes of MSH2, MSH3 and MSH6, the key proteins of the MMR pathway (M2KO, M3KO and M6KO respectively), thereby interfering with or reducing the functions of the MutSα and MutSβ complexes and the activity of the complex. The strength determines the editing efficiency of Prime editing in hPSCs. MSH2 deletion significantly improves the editing efficiency in base mismatching and IDL (indel loop) of 1 to 10 bp; MSH3 deletion improves the editing efficiency in IDL of 3 to 10 bp; MSH6 deletion improves editing efficiency in base mismatches and 1~3 bp IDL;
Prime editing system increased the overall editing efficiency by 50 times on hPSCs with MSH2, MSH3 and MSH6 knockout, while the deletion of MSH2, MSH3 and MSH6 proteins had a negative impact on hPSCs. Pluripotency only has a minor effect and maintains pluripotency similar to wild-type cells. By KO key genes of hPSCs, the editing efficiency of the PE system in human pluripotent stem cells can be effectively improved, providing reliable gene editing for in vitro stem cell therapy.
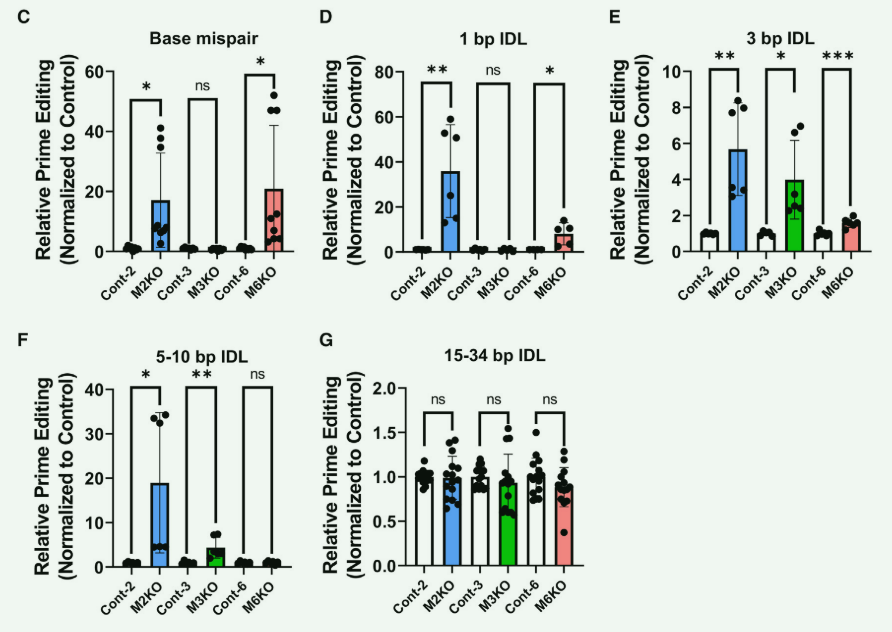
Figuere 2 Deletion of MSH2, MSH3 and MSH6 proteins improves the editing efficiency of Prime editing system in hPSCs
EDITGENE focuses on CRISPR technology and can provide gene knockout cell and PE point mutation cell generation services. We worked on more than 300 types of cells. Now we are having promotions for custom cell line service!
PEmax point mutation mesenchymal stem cells become a new treatment direction for hereditary primary hypogonadism
Hereditary primary hypogonadism (HPH) is caused by genetic mutations in the luteinizing hormone/chorionic gonadotropin receptor (LHCGR), a hormone involved in testosterone synthesis in Leydig cells. And HPH often impairs male’s sexual development and spermatogenesis.
Researchers established a new point mutation mouse model (LhcgrW495X) based on gene mutations related to HPH patients. They successfully corrected the in vivo mutations of mesenchymal stem cells (SLCs) of LhcgrW495X point mutation mice through lentiviral delivery of the PEmax system and effectively regenerated them. When it differentiate into Leydig cells, restore testosterone production, restart sexual development, restore spermatogenesis, and produce fertile offspring. The average correction rate within 7 days is 11.67%, and the average correction rate within 14 days is 23.42%. D eep sequencing of t he five potential off-target sites was performed and no detectable off-target editing was observed. The researchers found that gene-corrected SLCs in LhcgrW495X mice could differentiate into functional mesenchymal cells in vitro. Transplantation of mesenchymal stem cells cultured in vitro into LhcgrW495X point mutation mice significantly improved HPH symptoms. In the future, it is hoped that humanized SLCs can be used to treat human HPH.
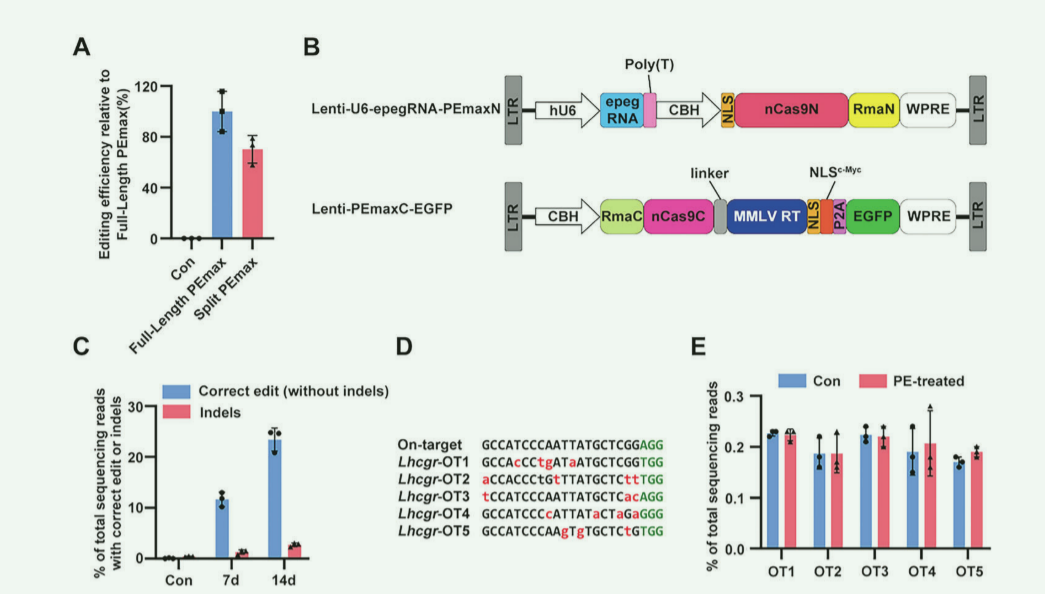
Figure 3 The PEmax system has high editing efficiency in W495X-SLCs, and no off-target genes were observed.
Based on PE (Prime Editing) technology, EDITGENE has comprehensively upgraded the Bingo™ point mutation platform. The success rate of point mutation far exceeds the traditional editing method. The point mutation cell line service is starting from $4500.
S equencing results of a p oint mutation cell clone
Contact Us
Email:info@editxor.com
Phone:833-2263234 (USA)
Recent Posts:
Prime editing 101:what is prime editing?
[Research Frontier] New trends in CRISPR detection: Monkeypox virus detection










![[Literature Review] A Novel Mechanism of Cisplatin Resistance in Osteosarcoma: CRISPR Screening Identifies Key Regulators in Organoid Models](/uploads/20250527/bL2GJjteMDvzmZys_53c82bdd67704fe0e159246934f924ee.png)
![[Quality Share] Decoding Point Mutations: A Comprehensive Guide to Three Common Construction Methods](/uploads/20250328/ESzk5OC49wpxIHVv_3cbfa5e98ea1d238127fe23c72b0f4b2.png)

Comment (4)