HES-KI Technology Platform
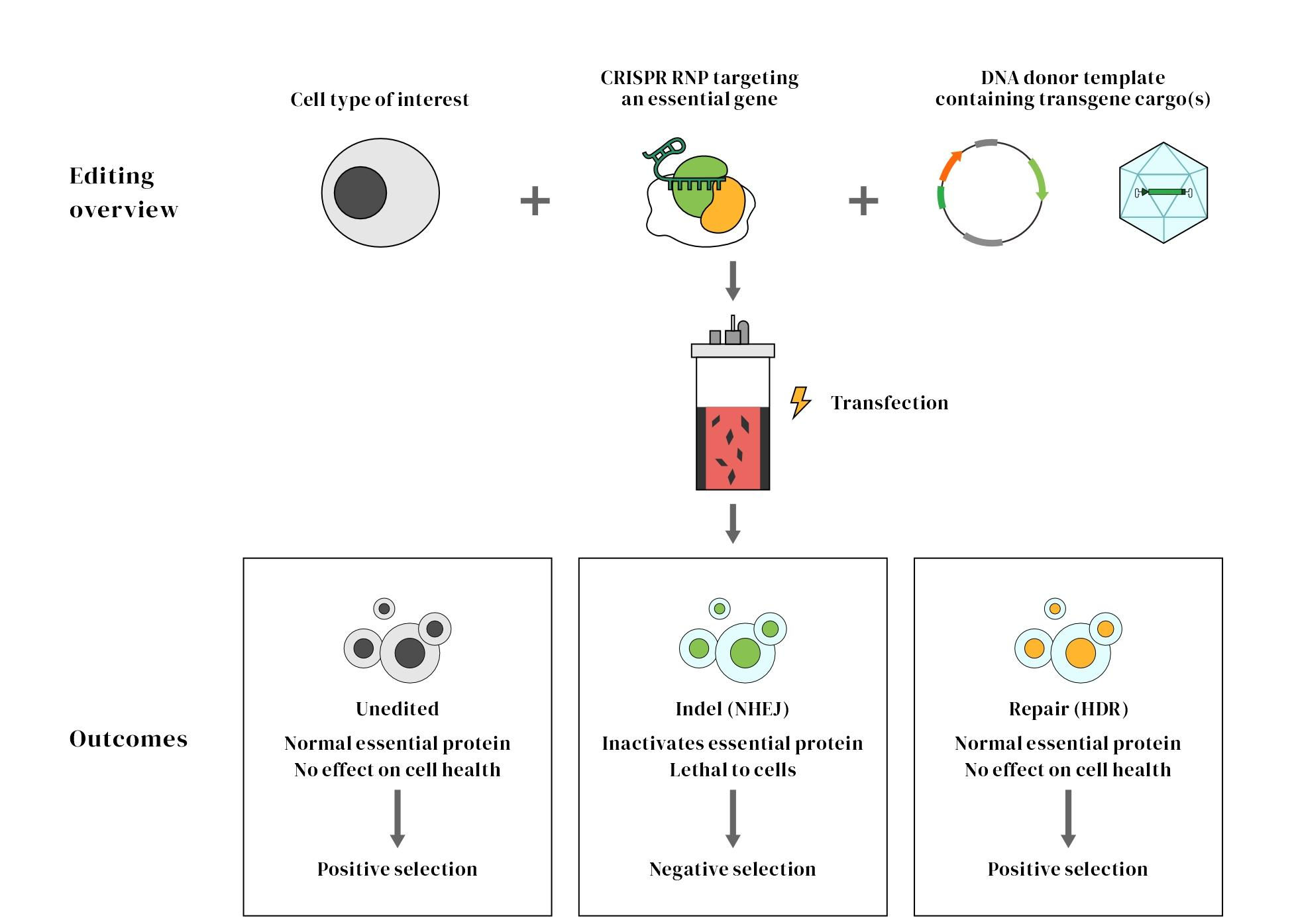
HES-KI ensures high-efficiency gene knock-in through a unique selection mechanism
Gene knock-in (KI) technology holds great promise in cell therapy and industrial biomanufacturing. However, conventional knock-in strategies often suffer from low efficiency, limiting their broader application in these fields. Current knock-in methods largely depend on homology-directed repair (HDR), but cells naturally favor non-homologous end joining (NHEJ) to repair double-strand breaks (DSBs), which leads to low integration efficiency. Among the existing platforms, CRISPR/HDR combined with AAV (adeno-associated virus) donor systems is frequently used and relatively efficient. However, AAV donors are prone to tandem insertions into the genome and come with limitations such as high cost, complex production, and limited cargo capacity. These challenges further restrict the widespread application of gene knock-in technologies.
HES-KI is a novel knock-in platform that targets the C-terminal exon region of essential genes for CRISPR editing. A knock-in template is designed such that successful integration restores the function of the essential gene and inserts the desired transgene. In contrast, cells with failed knock-ins—such as those repaired via NHEJ resulting in indels—lose the function of the essential gene and cannot survive. This creates a natural selection pressure that enriches for successfully edited cells.
Design Principle

Technical Advantages
• Stable and uniform expression: The expression of the target gene is highly stable and consistent in successfully knocked-in monoclonal cells.
• Multi-gene integration: Multiple genes can be integrated simultaneously, such as inserting multiple CAR genes to target a variety of tumor antigens, reducing tumor escape.
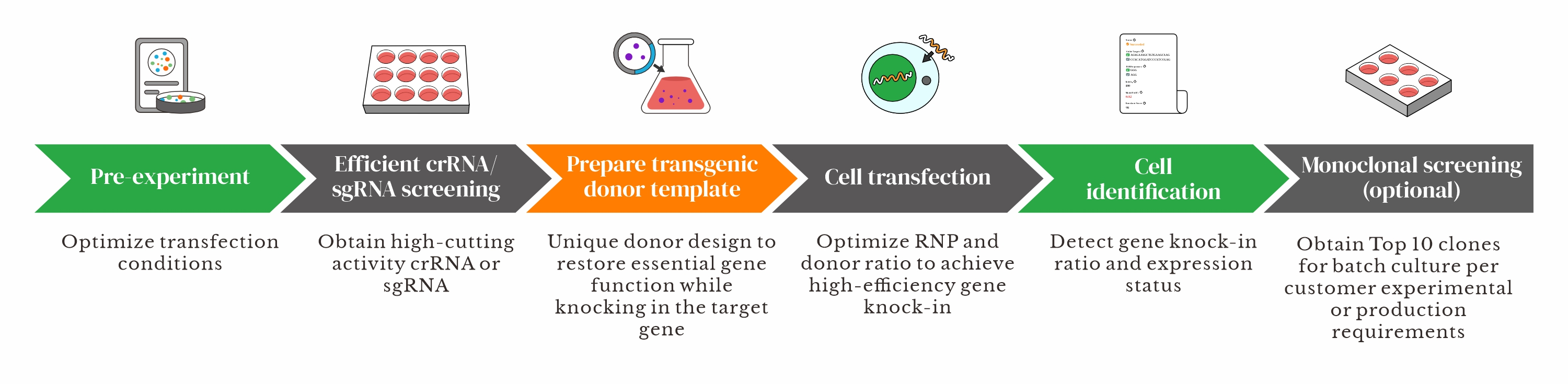
Workflow
HES-KI System Selection
Different important genes with varying expression levels can be selected as knock-in targets based on experimental needs. The appropriate CRISPR system and donor delivery method can be chosen based on the ease of cell transfection.
| Important Gene | CRISPR System | CRISPR Delivery Method | Form of Donor |
|---|---|---|---|
| GAPDH TUBB KIF11 TBP ...... |
Cas9-sgRNA | Plasmid | Plasmid |
| AsCas12a-crRNA | RNP | AAV |
Case Study
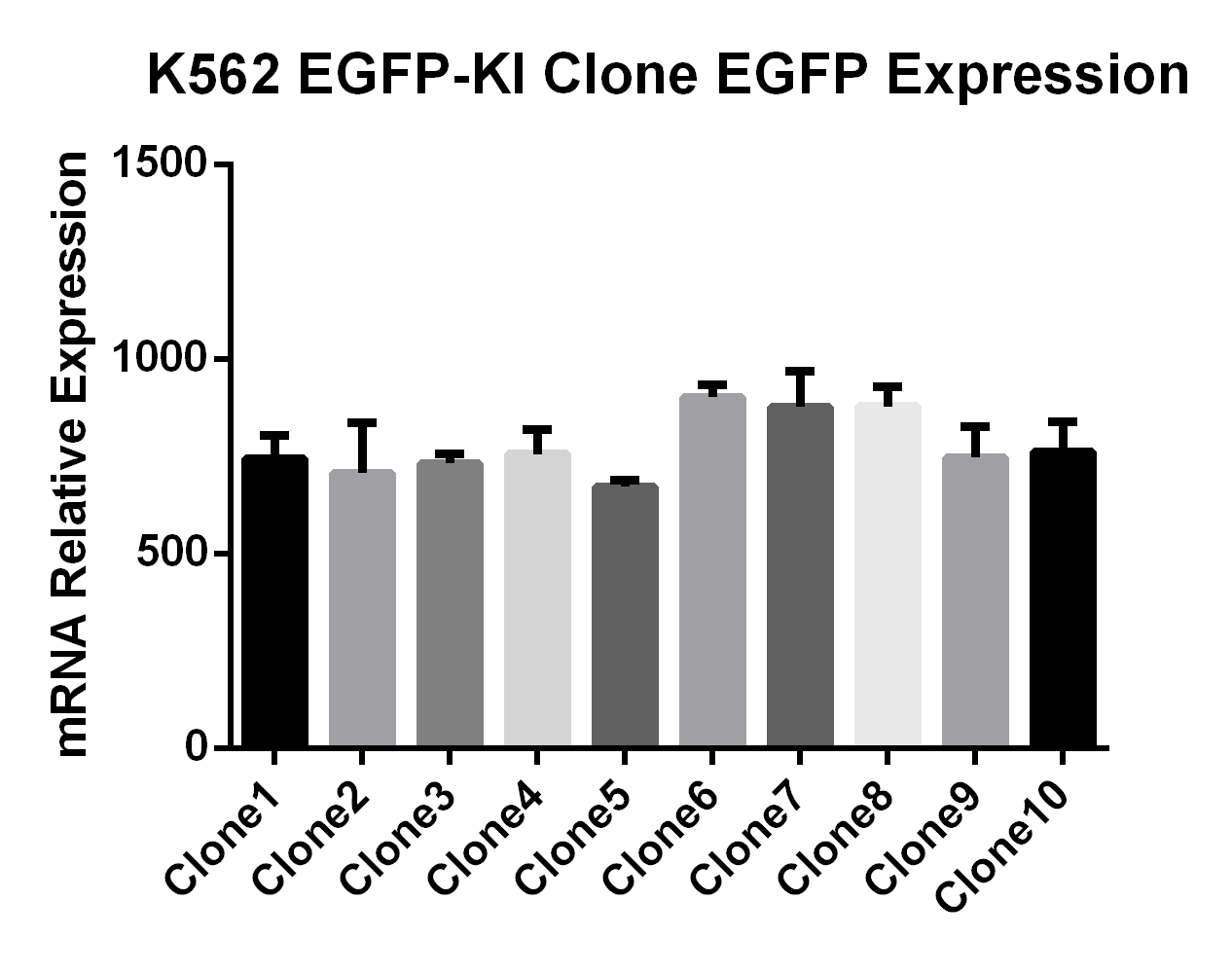
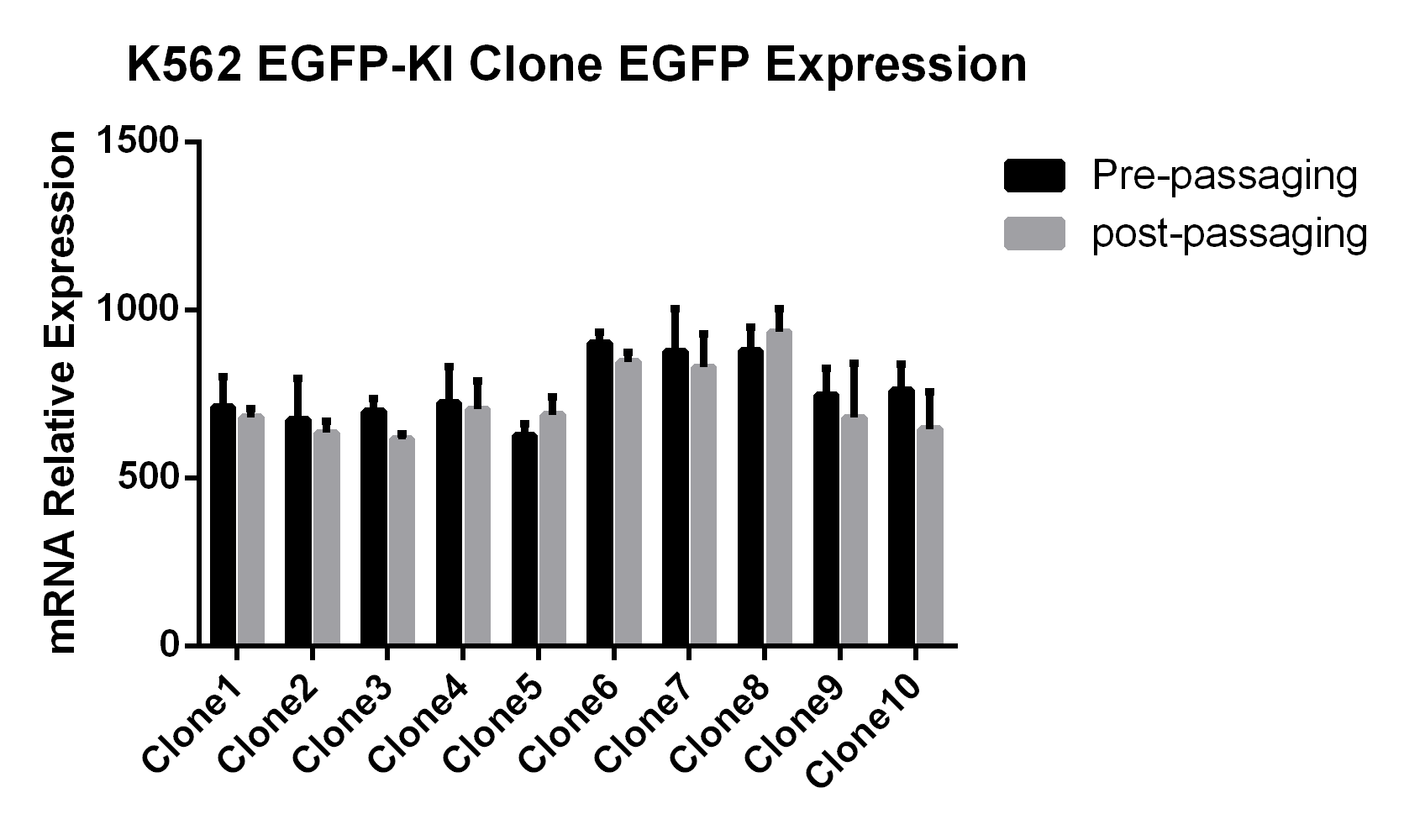
Objective: Insertion of EGFP at the C-terminal of the GAPDH gene in K562 cells
Design
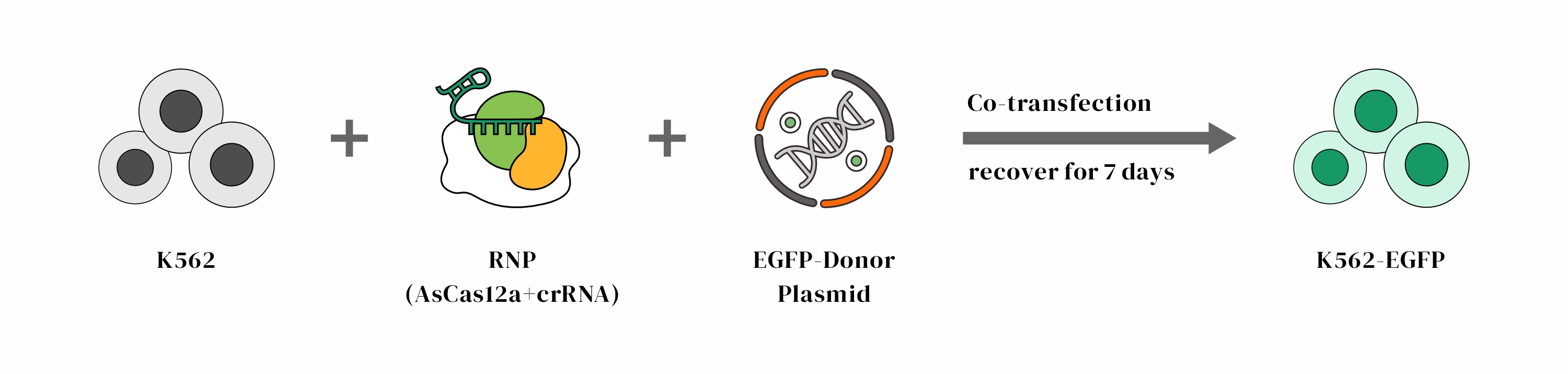
• Using the HES-KI technology, EGFP was inserted into the GAPDH gene in K562 cells, achieving a knock-in efficiency of 37% in multiple clones without the use of resistance selection.

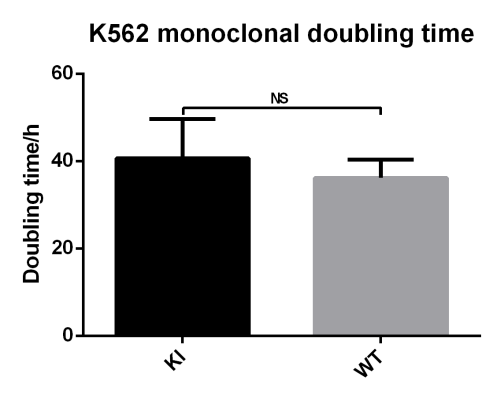
• There was no significant difference in the doubling time between K562 EGFP-KI monoclonal cell lines and WT cells, and the growth of K562 cells was unaffected by the EGFP insertion.
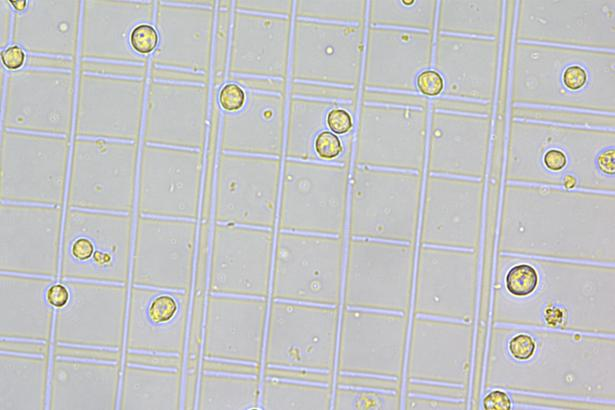
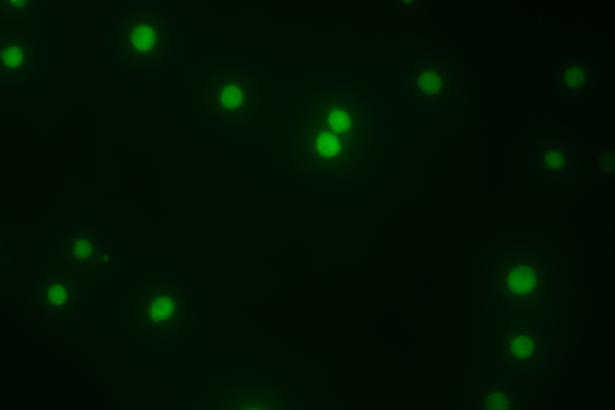
| K562 EGFP-KI polyclonal cells | |
 |
 |
| K562 EGFP-KI monoclonal cells | |
 |
 |
Objective: Insertion of EGFP at the C-terminal of the GAPDH gene in 293T and CHO-K1 cells
Design:
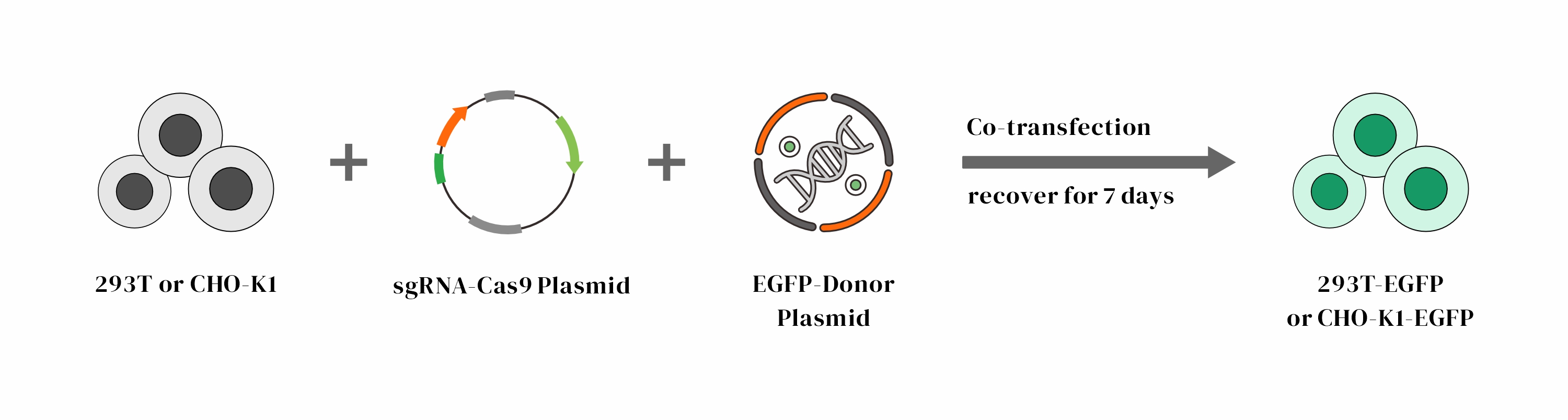 Result:
Result: 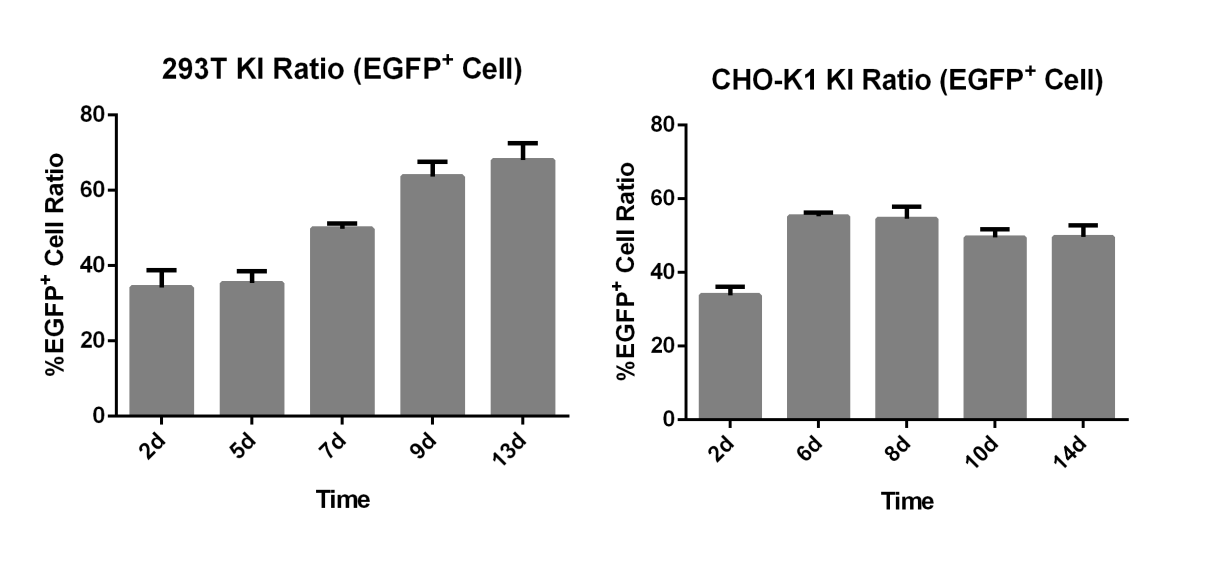
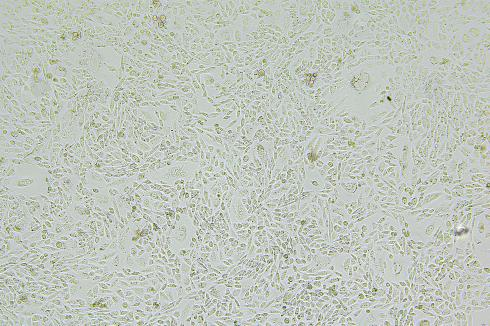
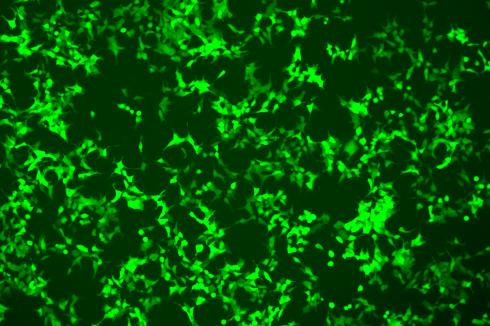
• Images of 293T and CHO-K1 EGFP-KI polyclonal cells.
| 293T | CHO-K1 |
 |
 |
 |
 |
Objective: Successful knock-in of GFP and mCherry genes at the C-terminal of the GAPDH gene in IPSC cells using AsCas12a-mediated HES-KI technology.
Design:

Result:

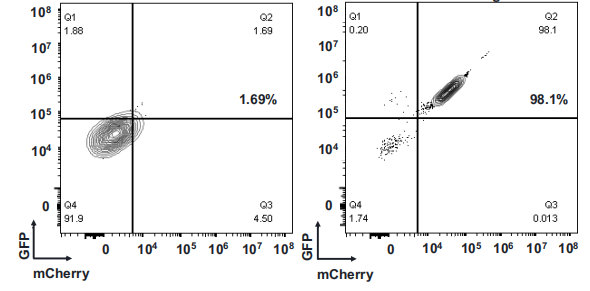
Objective: Successful knock-in of a 5.2 kb large gene fragment at the C-terminal of the GAPDH gene in IPSC cells using AsCas12a-mediated HES-KI technology.
Design:
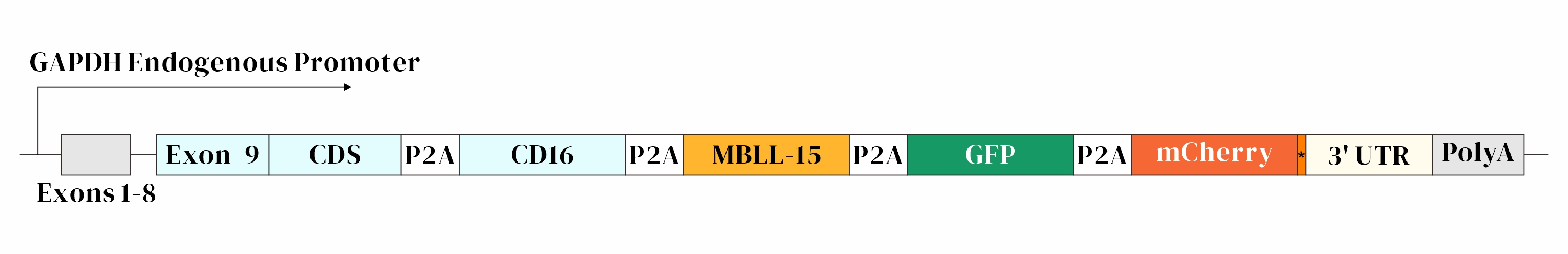
Result:










![[Literature Review] A Novel Mechanism of Cisplatin Resistance in Osteosarcoma: CRISPR Screening Identifies Key Regulators in Organoid Models](/uploads/20250527/bL2GJjteMDvzmZys_53c82bdd67704fe0e159246934f924ee.png)
![[Quality Share] Decoding Point Mutations: A Comprehensive Guide to Three Common Construction Methods](/uploads/20250328/ESzk5OC49wpxIHVv_3cbfa5e98ea1d238127fe23c72b0f4b2.png)

Comment (4)