[Client Publication] EDITGENE's Point Mutation Cells Illuminate the Role of TTN Mutations in DNA Repair and Immune Infiltration
HCT116 and SW837 cells
Rectal adenocarcinoma (READ) is a challenging malignancy often treated with surgery, chemotherapy, and radiotherapy. Radiotherapy plays a vital role, especially for patients with locally advanced disease. Yet, not all patients respond the same way, making it crucial to uncover the molecular factors that influence sensitivity to radiation. The TTN gene, known for encoding titin—a protein essential for muscle elasticity—has been linked to various diseases. However, its role in how tumors respond to radiotherapy has remained a mystery.
Recently, Chinese researchers have published a paper in journal Clinical and Translational Medicine, titled “Titin gene mutations enhance radiotherapy efficacy via modulation of tumour immune microenvironment in rectum adenocarcinoma” Their study reveals how TTN mutations improve radiosensitivity by affecting DNA damage repair and immune cell infiltration. They also propose TTN mutation as a potential biomarker to predict radiotherapy response.
EDITGENE is honored to have supported this cutting-edge research by providing HCT116 and SW837 cells. Below is a brief overview of the publication.
Original link:https://doi.org/10.1002/ctm2.70123
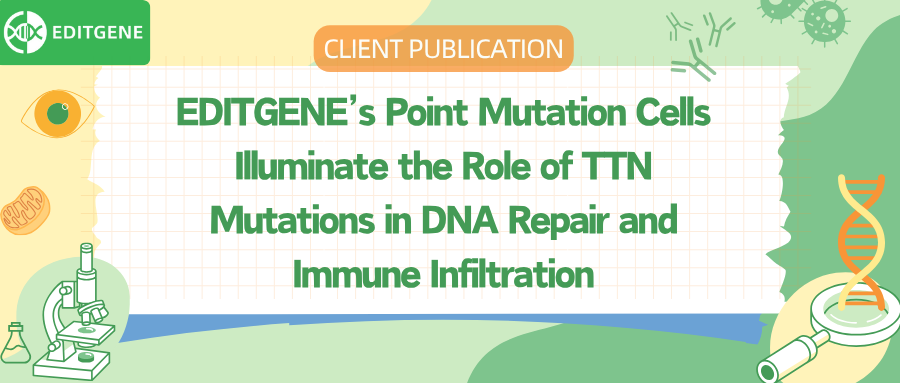
By analyzing gene expression and mutation data from public databases, the researchers found that TTN mutations occur frequently in rectal adenocarcinoma (READ) and show a significant association with the tumor immune microenvironment.
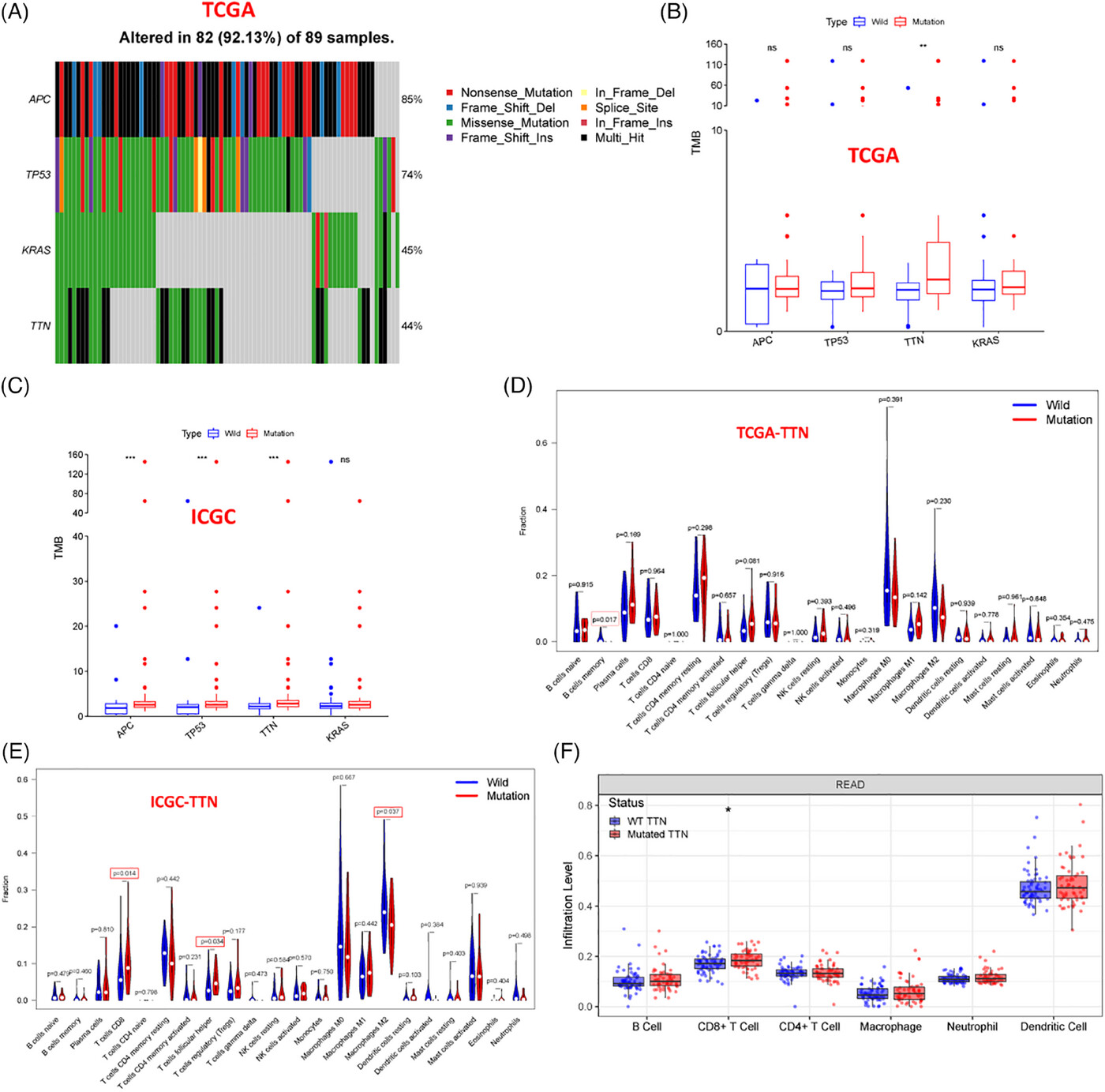
Figure 1: Correlation analysis of TTN mutations with tumor mutation burden (TMB) and immune cell infiltration in READ.
TTN knockout and mutant cell lines ( HCT116 and SW837 ) were generated using CRISPR/Cas9 technology. The radiosensitivity of TTN-mutant cells was evaluated through colony formation assays, while Western blotting was used to assess the expression of apoptosis-related proteins.
The results showed that TTN-mutant cells had significantly reduced colony-forming ability after radiation, exhibited higher levels of DNA damage, and lower expression of DNA repair proteins—indicating increased sensitivity to radiotherapy.
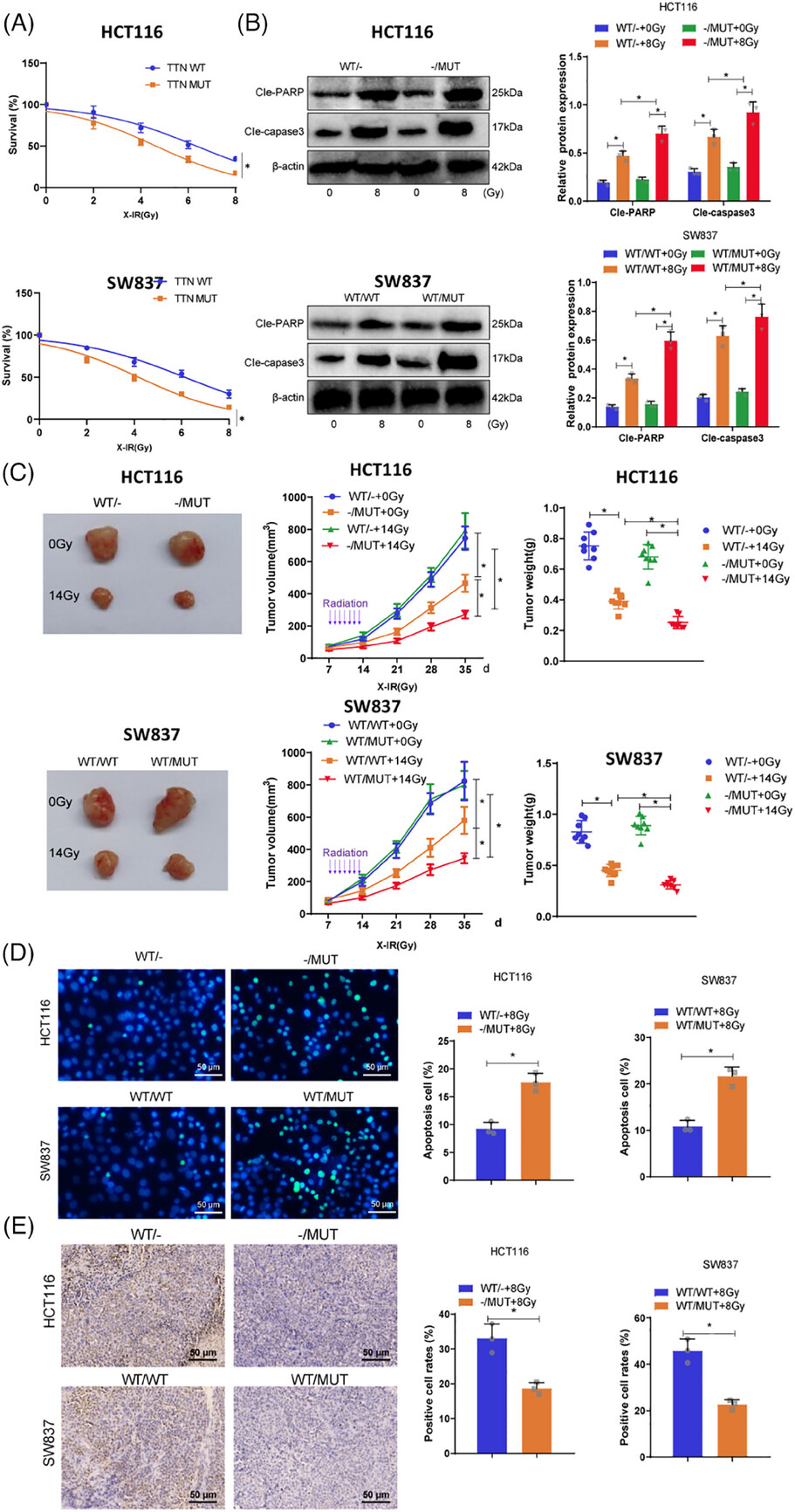
Figure 2: Assessment of TTN mutation impact on radiosensitivity in vitro and in vivo.
Researchers established subcutaneous tumor models using TTN wild-type and mutant cells in BALB/c nude mice and C57BL/6 mice. Radiotherapy was administered, and tumor growth was monitored over time. Immunohistochemistry (IHC) and flow cytometry were used to analyze CD4⁺ and CD8⁺ T cell infiltration in tumor tissues.
The results showed that TTN-mutant tumors exhibited significantly greater growth suppression following radiotherapy, accompanied by markedly increased infiltration of CD4⁺ and CD8⁺ T cells.
Figure 3. Evaluation of immune stimulation in subcutaneous xenograft mouse models following radiotherapy in TTN knockout tumors.
To evaluate the predictive value of TTN mutations for radiotherapy response, tumor samples from 32 LARC patients who received radiotherapy were collected. ANKRD1 expression was assessed by immunohistochemistry (IHC), and CD4⁺ and CD8⁺ T cell levels were analyzed via immunofluorescence. Kaplan-Meier survival analysis was conducted to examine the association between TTN mutation status, ANKRD1 expression, T cell infiltration density, and disease-free survival (DFS).
The results showed that radiotherapy led to a significant decrease in ANKRD1 expression and a marked increase in CD4⁺ and CD8⁺ T cell infiltration. Patients with TTN mutations, low ANKRD1 expression, and high densities of CD4⁺ and CD8⁺ T cells had significantly longer 3-year DFS.
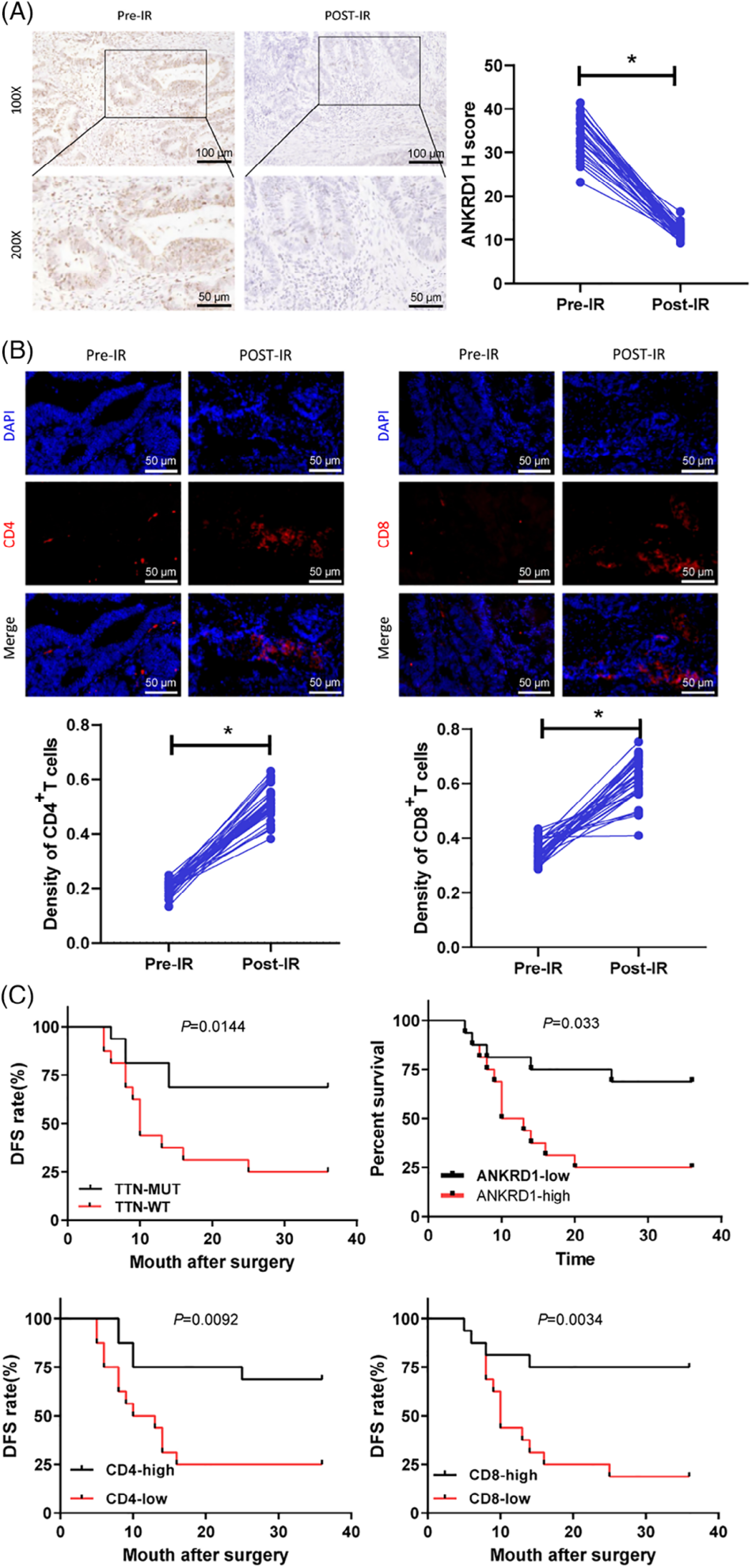
Figure 4. TTN mutation-driven immunogenic activation and its correlation with radiotherapy outcomes in READ patients.
This study highlights the role of TTN mutations in enhancing radiosensitivity in rectal adenocarcinoma and suggests their potential as predictive biomarkers for radiotherapy response. Moreover, the findings demonstrate that modulating the tumor immune microenvironment can significantly improve radiotherapy outcomes. Together, these insights lay the groundwork for developing more personalized radiotherapy strategies and identifying novel targets for immunotherapy.
















