Alzheimer’s disease (AD) is a progressive neurodegenerative disorder marked by β-amyloid (Aβ) accumulation, neuronal loss, and neuroinflammation. Traditional AD animal models—typically transgenic mice overexpressing familial AD mutations—have limitations due to artificial gene expression levels and immunogenic backgrounds, which hinder their ability to accurately reflect human immune responses. This often results in a gap between preclinical findings and clinical trial outcomes. To bridge this gap, more physiologically relevant models that better mimic the pathology and immune landscape of human AD are urgently needed.
In February 2025, Alzheimer’s & Dementia published a study titled "Amyloid precursor protein and presenilin-1 knock-in immunodeficient mice exhibit intraneuronal Aβ pathology, microgliosis, and extensive neuronal loss." Led by Howard E. Gendelman’s team, the research introduced AD-related mutations in the APP and PS1 genes into immunodeficient mice. This knock-in model successfully reproduced key AD features, including intraneuronal Aβ pathology, microglial activation, and significant neuronal loss. The model offers a valuable new tool for studying how immune mechanisms contribute to AD in a setting that more closely mirrors human disease.
Original link: https://doi.org/10.1002/alz.70084
For the first time, APP and PS1 mutations were introduced into immunodeficient NOG mice using a knock-in strategy. Gene expression is driven by endogenous promoters, avoiding the non-physiological overexpression commonly seen in traditional transgenic models.
2.Intraneuronal Aβ pathology
The model replicates early-stage intraneuronal Aβ accumulation and its associated neurotoxicity, addressing a major limitation of conventional models that primarily feature extracellular plaques.
3.Extensive neuronal loss
This model is the first to recapitulate widespread neuronal loss and brain atrophy in mice—hallmarks of human AD that are typically absent or limited in traditional models, which often show only localized neurodegeneration.
4.Potential for humanized immunity
The immunodeficient background allows for the reconstitution of a human immune system, offering a new platform to investigate the role of human immune responses in Alzheimer’s disease pathogenesis.
CRISPR/Cas9 knock-in is a precise gene editing method based on the CRISPR system. It enables targeted modifications to the genome by inserting or replacing specific DNA sequences at defined loci. With its high specificity, accuracy, and flexibility, CRISPR/Cas9 knock-in technology has become a fundamental tool for gene function studies, disease model development, and gene therapy applications.
丨.Generation of Knock-In Mice
1. Gene design Researchers introduced the Swedish double mutation (K670N/M671L) into exon 16 of the mouse App gene, along with partial humanization (G676R/F681Y/R684H). For the Ps1 gene, the M146V mutation was inserted into exon 5, together with a humanizing substitution (I145V).
2.CRISPR editing
Specific sgRNAs were designed using the CRISPR Web tool, with careful selection to minimize off-target effects. Fertilized eggs were collected from female NOD mice. A mixture of Cas9 protein, sgRNAs, and humanized donor DNA was microinjected into the zygotes. Targeted knock-in was achieved via homology-directed repair (HDR). The edited embryos were then transferred into pseudopregnant females and carried to term.
3.Genotyping and homozygosity
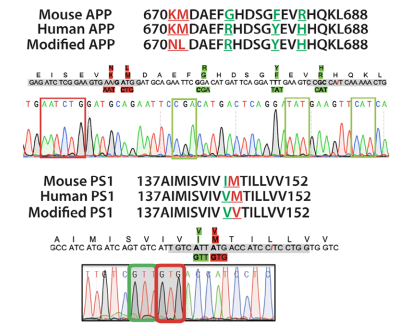
Genotyping: Ear tissue was collected at weaning for genomic DNA extraction using alkaline lysis. Target regions were amplified by PCR with specific primers, and purified PCR products were confirmed via Sanger sequencing to verify the presence of APP and PS1 mutations along with humanized sequences.
Establishing homozygosity: Heterozygous knock-in mice were backcrossed to NOG mice to establish homozygous lines for individual knock-ins—APP (NA) and PS1 (NPS). These NA and NPS mice were then crossed to generate homozygous double knock-in APP/PS1 (NAPS) mice.
Figure 1. Sanger sequencing confirmed the successful replacement of pathogenic mutations (red box) and humanized nucleotide sequences (green box) in exon 16 of the mouse App gene and exon 5 of the Ps1 gene.
II.Pathological Phenotype Validation
1. Protein expression analysis
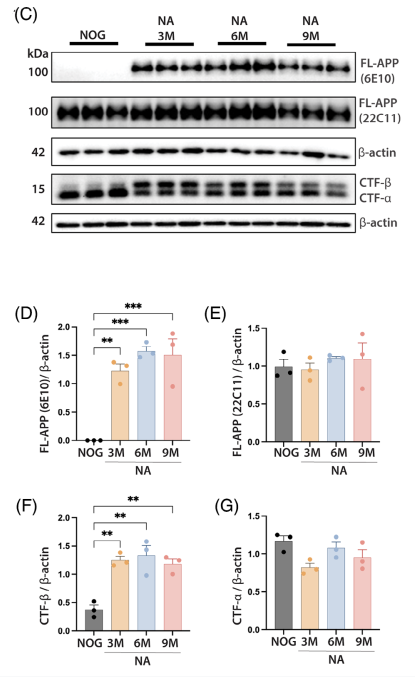
Western blotting was used to detect FL-APP (6E10 antibody) and CTF-β fragments in cortical tissue from the mice, confirming abnormal APP cleavage induced by the mutations.
2.ELISA
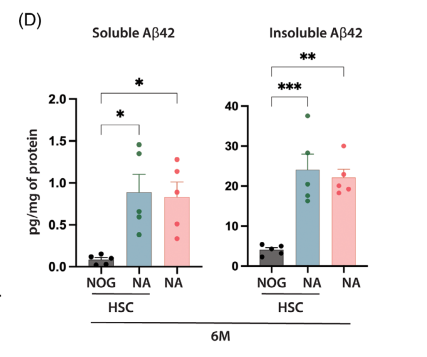
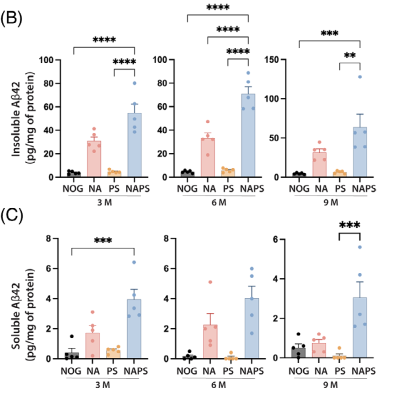
Quantification of soluble and insoluble Aβ42 levels showed that NAPS mice had twice the amyloid burden of NA mice.
3.Histopathology
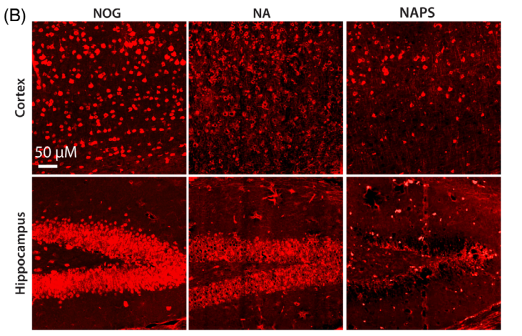
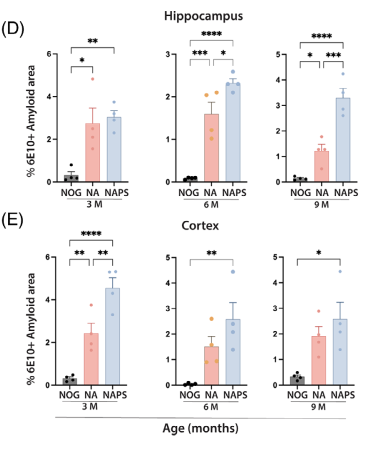
3.1 Immunohistochemistry (IHC): 6E10 antibody staining revealed intracellular Aβ deposition in the cortex and hippocampus of 3-month-old mice, with worsening accumulation as they aged.
3.2 Neuronal loss and brain atrophy (NeuN/MAP2 staining): Significant loss of cortical and hippocampal neurons was observed in both NA and NAPS mice.
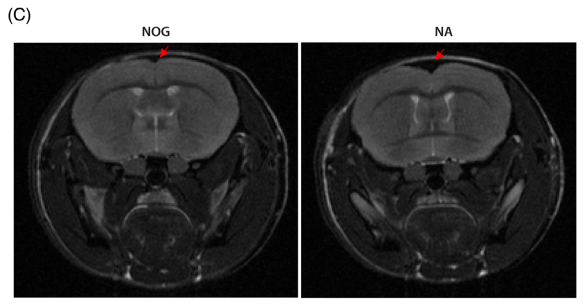
3.3 MRI scans: At 12 months of age, NA mice showed reduced cortical volume and expanded ventricles.
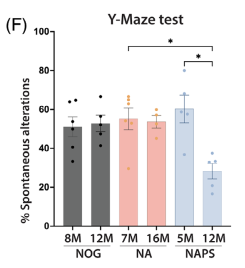
3.4 Behavioral tests (Y-maze): At 12 months of age, NAPS mice exhibited a decreased spontaneous alternation rate, suggesting impaired spatial memory.
III. Human Immune System Reconstitution
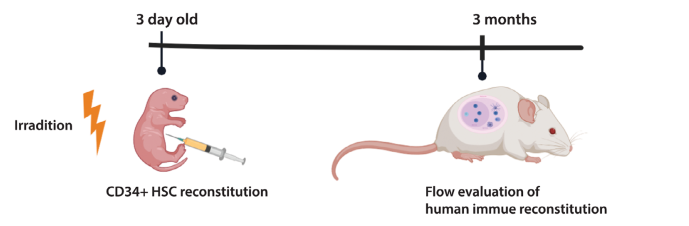
Newborn NA mice (0-3 days old) were irradiated with 1 Gy X-rays for 4 hours and then injected with human CD34+ hematopoietic stem cells (HSCs) into the liver. After 12 weeks, flow cytometry of peripheral blood from both NA and NOG control mice confirmed the successful reconstitution of human immune cells, including CD45+, CD3+, and CD19+ cells.
Results showed no significant differences between HSC-reconstituted NOG and NA mice, and HSC reconstitution did not significantly alter Aβ burden.
Figure 2. Schematic of human immune system reconstitution in NA mice.
Figure 3. HSC reconstitution did not significantly alter Aβ burden.
Summary and Outlook
This study generated an APP/PS1 knock-in mouse model with an immunodeficient background using CRISPR-Cas9 knock-in technology. This model successfully replicates key AD pathological features, including intraneuronal Aβ deposition, microglial activation, and extensive neuronal loss, and offers potential for human immune system reconstitution. Despite limitations in plaque formation and behavioral deficits, the model presents significant potential for studying pathological mechanisms and evaluating immunotherapies, providing a more human-like tool for AD research.
EDITGENE specializes in customized gene knockout cell line development, delivering powerful tools that help researchers unlock critical mechanisms and drive discoveries from bench to bedside.Ready to accelerate your gene editing research? Contact us today to tailor a solution for your study. EDITGENE– making target validation more rapid and more precise.
Recent Blogs
Follow us on social media





Contact us
+ 833-226-3234 (USA Toll-free)
+1-224-345-1927 (USA)
info@editxor.com