【Literature Review】EZH2 Knockout Mouse Model Sheds Light on Ferroptosis Mechanisms in Pulpitis
knockout mouse model

Summary:
A recent study successfully established an EZH2 knockout mouse model using precise gene editing technology, revealing the critical role of EZH2 in ferroptosis associated with pulpitis. This model provides strong support for in-depth investigations into the pathogenesis of pulpitis and the development of novel therapeutic strategies.
At the forefront of dental research, gene editing technology is increasingly becoming a vital tool for uncovering disease mechanisms. In 2025, the International Endodontic Journal published a highly impactful study titled:
“EZH2 knockout in mice activates STAT3 signalling via STAT3 methylation and modulates ferroptosis in pulpitis-affected dental pulp vascular endothelial cells: A laboratory investigation.”
Led by Dr. Weilin Zhou and Dr. Weili Huang from the Affiliated Stomatological Hospital of Sun Yat-sen University, the research team constructed an EZH2 knockout (KO) mouse model to explore the role of Enhancer of Zeste Homolog 2 (EZH2) in pulpitis. They focused on how EZH2-mediated methylation of Signal Transducer and Activator of Transcription 3 (STAT3) regulates ferroptosis in dental pulp vascular endothelial cells.
This groundbreaking study not only identifies a promising new therapeutic target for treating pulpitis but also opens new avenues for investigating inflammation-related cell death mechanisms.
Background and Objective
Pulpitis is a common dental inflammatory condition, typically triggered by bacterial infection, dental caries, or traumatic injury. If left untreated, it can lead to irreversible damage to the dental pulp and eventual necrosis. In recent years, ferroptosis—a novel, iron-dependent form of regulated cell death—has been increasingly recognized for its role in inflammatory diseases. However, its involvement in the pathogenesis of pulpitis remains unclear. To elucidate the role of Enhancer of Zeste Homolog 2 (EZH2) in ferroptosis associated with pulpitis, the research team established a conditional EZH2 knockout mouse model.
Materials and Methods
1.Construction of the EZH2 Knockout Mouse Model
Using CRISPR/Cas9 gene editing technology, the researchers generated EZH2^fl/fl Cre^+/− mice in which EZH2 was specifically knocked out. This model enabled the direct investigation of EZH2’s function in dental pulp inflammation. Methyl-Capture Sequencing was employed to evaluate the effects of EZH2 deletion on DNA methylation profiles in pulp tissues.
2.In Vitro Cellular Experiments
To mimic dental pulp vascular endothelial cells, EOMA endothelial cells were used and stimulated with lipopolysaccharide (LPS) to induce an inflammatory microenvironment. The study further explored the regulatory function of the EZH2/STAT3 signaling axis in ferroptosis under these conditions.
3.Bioinformatics Analysis
Public databases were utilized to identify differentially expressed genes (DEGs) and associated changes in DNA methylation relevant to pulpitis progression.
Results
1.Impact of EZH2 Knockout on DNA Methylation
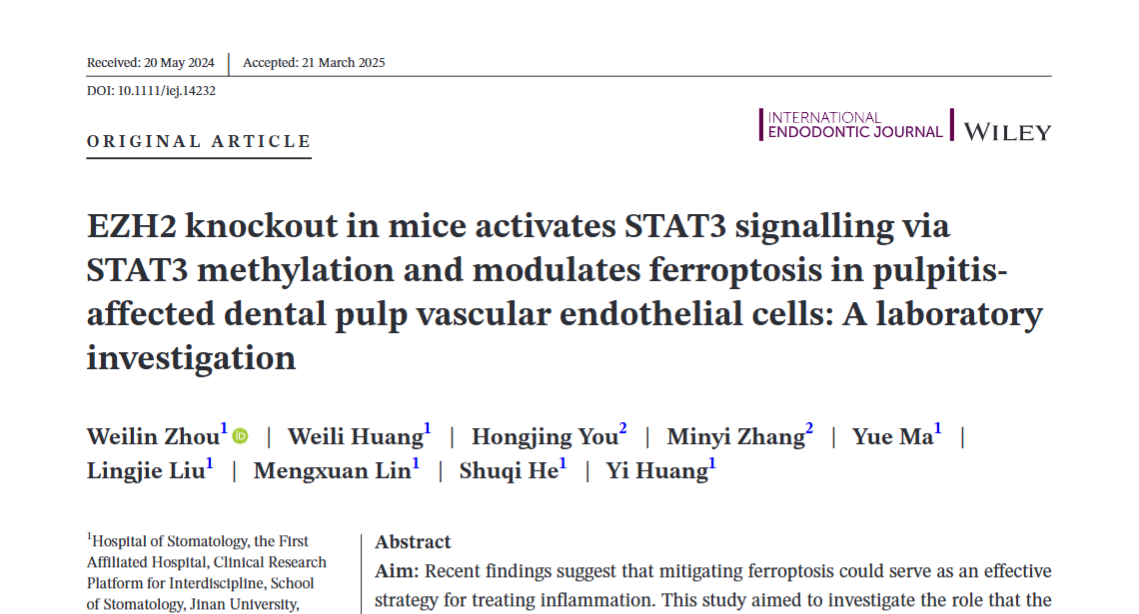
Methylation profiling revealed that EZH2 deletion resulted in 2,501 differentially methylated regions (DMRs) within dental pulp tissues, including 555 upregulated genes and 1,946 downregulated genes. These DMRs were predominantly enriched in biological processes such as phosphatidic acid transport, monocyte aggregation, and cytokine production—all of which are closely related to ferroptosis mechanisms.
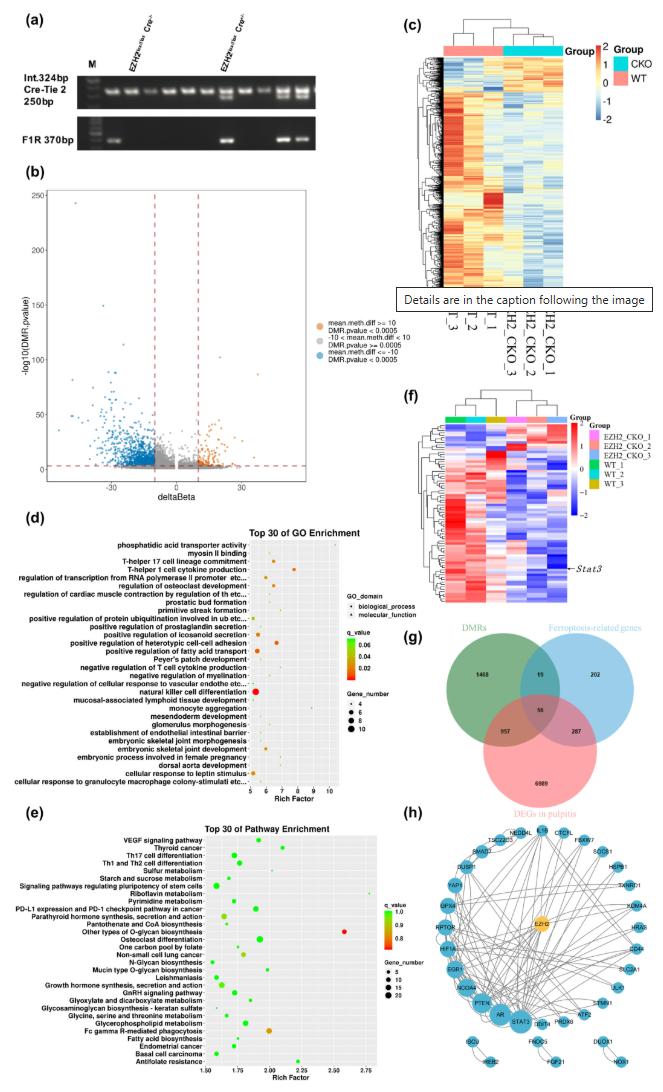
Figure 1: Methylation Capture Sequencing Analysis Reveals Gene Expression Changes and Pathway Enrichment Related
to Pulpitis and Ferroptosis.
2.LPS-Induced Ferroptosis in EOMA Cells
In LPS-stimulated EOMA cells, classical ferroptosis markers—including reactive oxygen species (ROS) levels, glutathione (GSH) content, and GPX4 expression—exhibited significant alterations. Treatment with EZH2 inhibitor GSK126 further intensified these ferroptosis-related changes, suggesting a regulatory role for EZH2 in modulating ferroptotic cell death during inflammation.
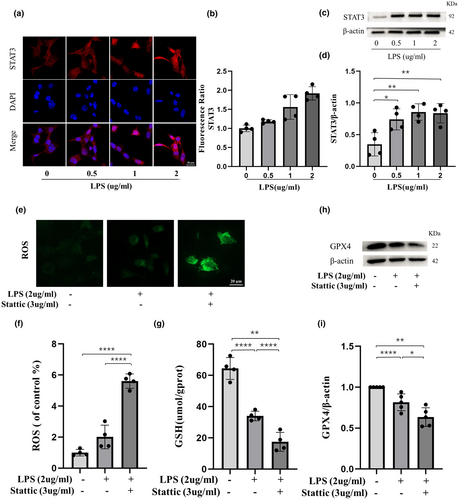
Figure 2. Effects of Lipopolysaccharide (LPS) and Static Stimulation on STAT3 and Ferroptosis-Related Markers.
3.The regulatory role of EZH2/STAT3 axis
EZH2 knockout led to a marked reduction in STAT3 and its phosphorylated form (p-STAT3), while the expression of GPX4—a key ferroptosis suppressor—increased. These findings suggest that EZH2 regulates ferroptosis through methylation-dependent modulation of the STAT3 signaling pathway.
Figure 3:Effects of GSK126 Treatment and Lentiviral Transduction on STAT3 and GPX4 Expression.
Conclusion
This study, through the construction of an EZH2 knockout mouse model, uncovers the critical role of EZH2 in ferroptosis associated with pulpitis. EZH2 modulates the expression of ferroptosis-related genes by regulating the methylation of STAT3, highlighting a novel epigenetic mechanism in the inflammatory response of dental pulp. These findings not only provide a robust in vivo tool for investigating the pathogenesis of pulpitis but also offer a theoretical foundation for developing therapeutic strategies targeting ferroptosis.
Summary and Outlook
The successful establishment of an EZH2-deficient mouse model provides a novel perspective for studying ferroptosis mechanisms in pulpitis. Future studies may explore the broader role of the EZH2/STAT3 axis in other inflammatory diseases and investigate the clinical potential of EZH2-targeting therapeutics. Integration with clinical sample analyses will be essential to translate these findings into effective treatment strategies for pulpitis and related conditions.
Bridging Research with Capability: Supporting Your Mouse Model Needs
The findings from this study underscore the value of precisely engineered knockout mouse models in uncovering disease mechanisms such as ferroptosis in pulpitis. At EDITGENE , we specialize in the custom generation of mouse models —including gene knockouts, knock-ins, point mutations, and complex multi-locus edits—to support advanced biomedical research using TurboMice™ Platform . Leveraging CRISPR/Cas9 and conditional allele systems, our services offer tailored solutions for academic, biotech, and pharmaceutical teams aiming to explore gene function, validate targets, or model human disease in vivo. Whether you're seeking EZH2 knockout mice, point mutations like STAT3 、 D661Y, or large-fragment insertions, EDITGENE provides end-to-end support from design to validation. Partner with us to accelerate your next discovery.
Recent Blogs
- 1. [Literature Review] CRISPR Screening Reveals the Critical Role of NUS1 in Prostate Cancer Survival and Growth
- 2. [Literature Review]EDITGENE Supports Research Team in Uncovering New Mechanism of Macrophage Polarization Regulated by Dental Pulp Stem Cells
- 3. [Quality Share]Cracking the Delivery Puzzle: How Can Gene Editing Precisely Reach Target Cells?
Follow us on social media
Contact us
+ 833-226-3234 (USA Toll-free)
+1-224-345-1927 (USA)
info@editxor.com
















