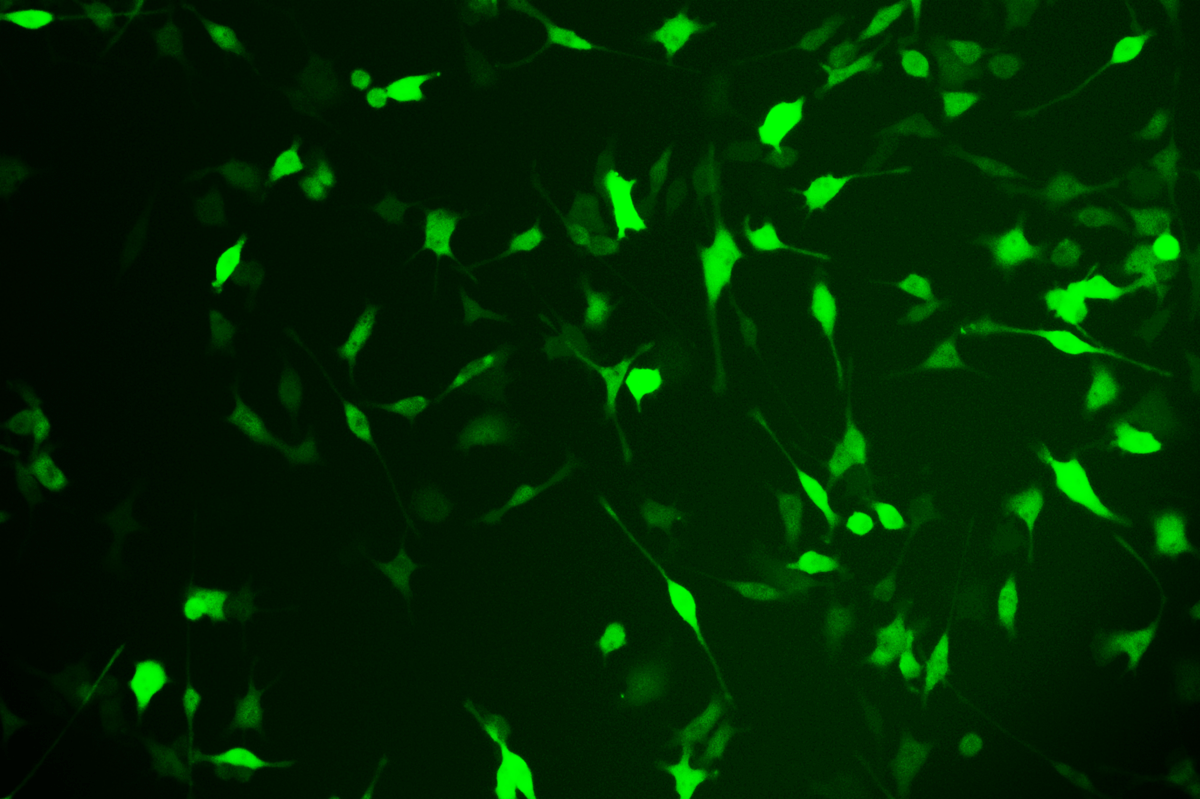
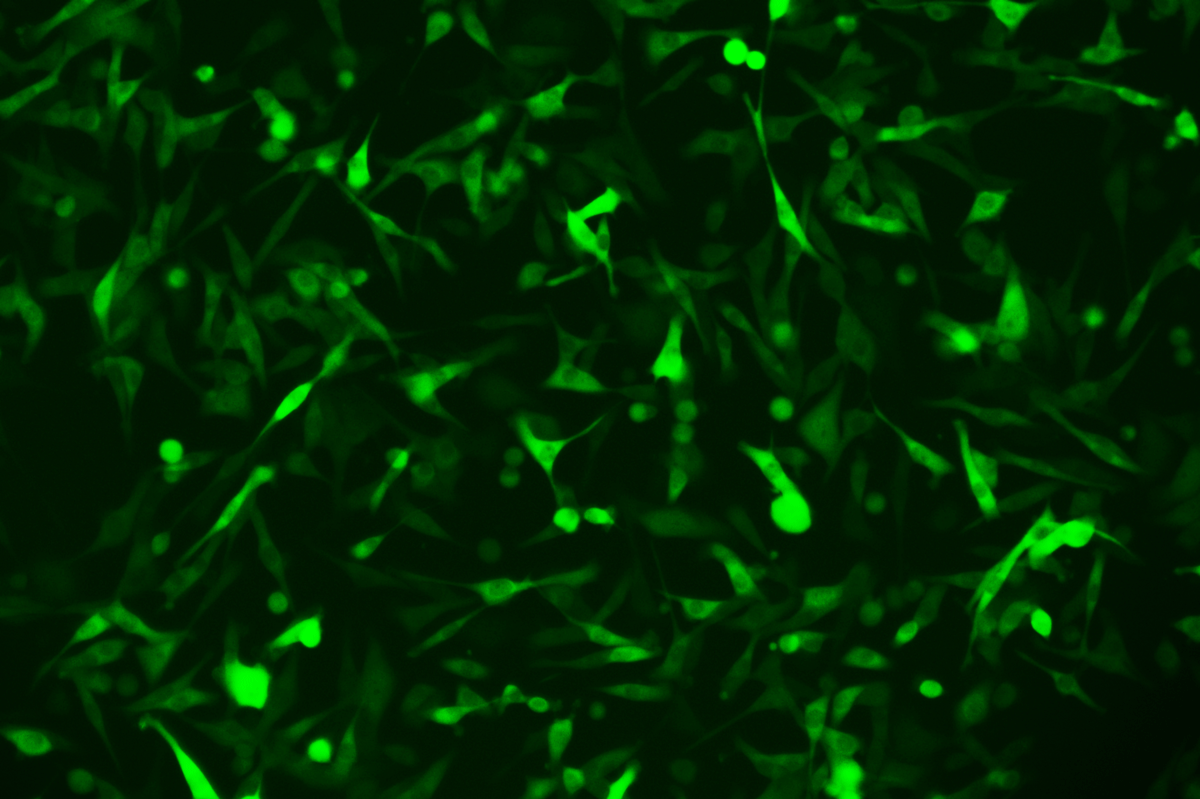
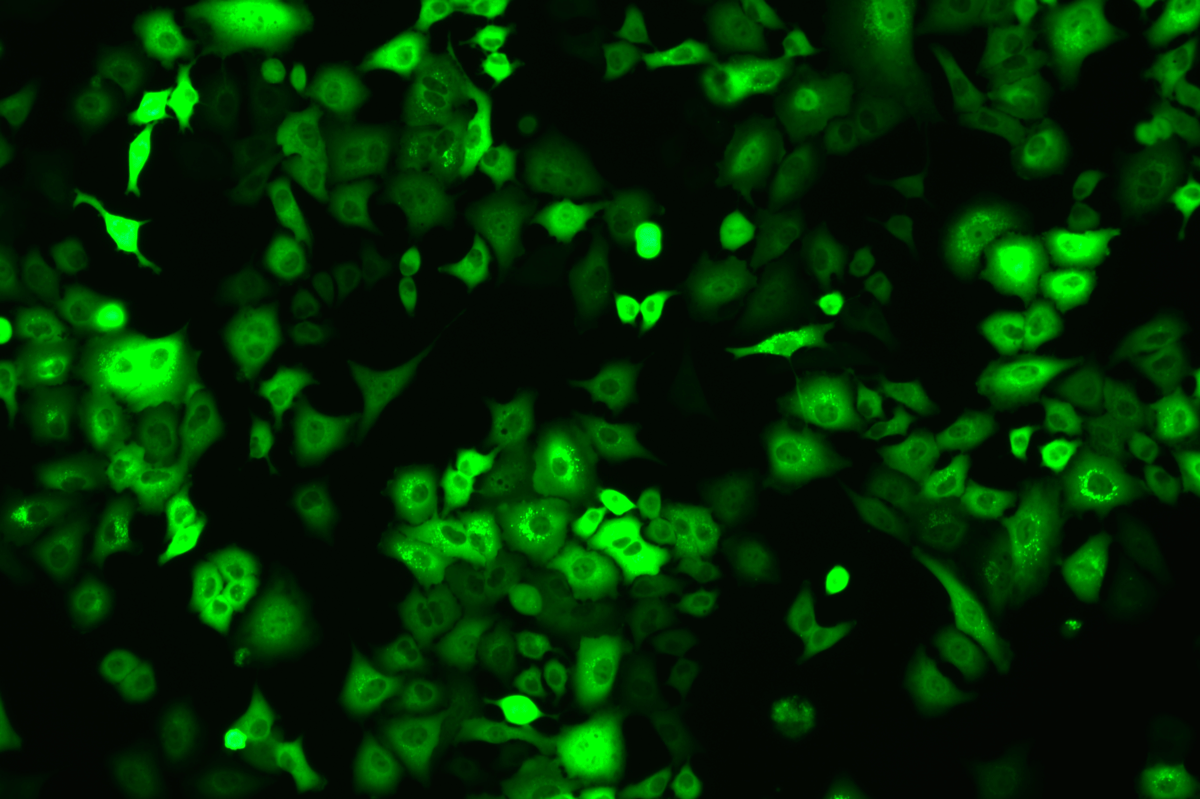
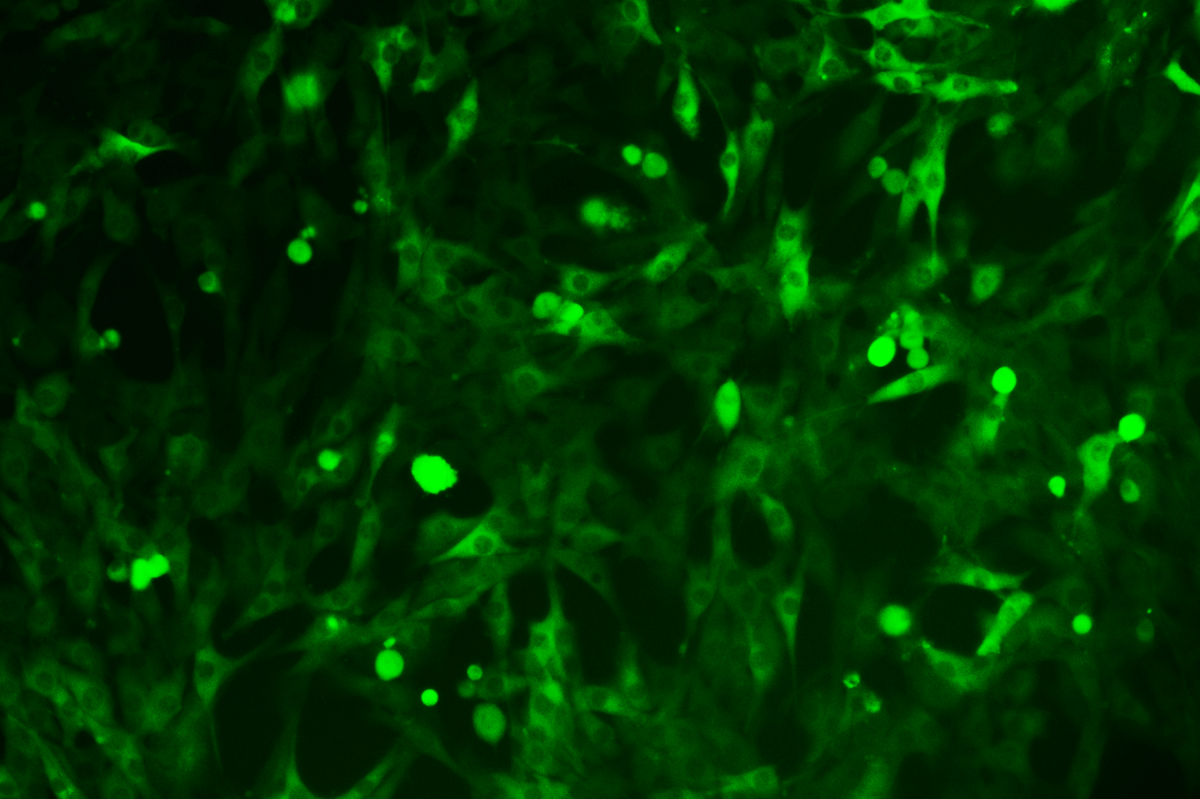
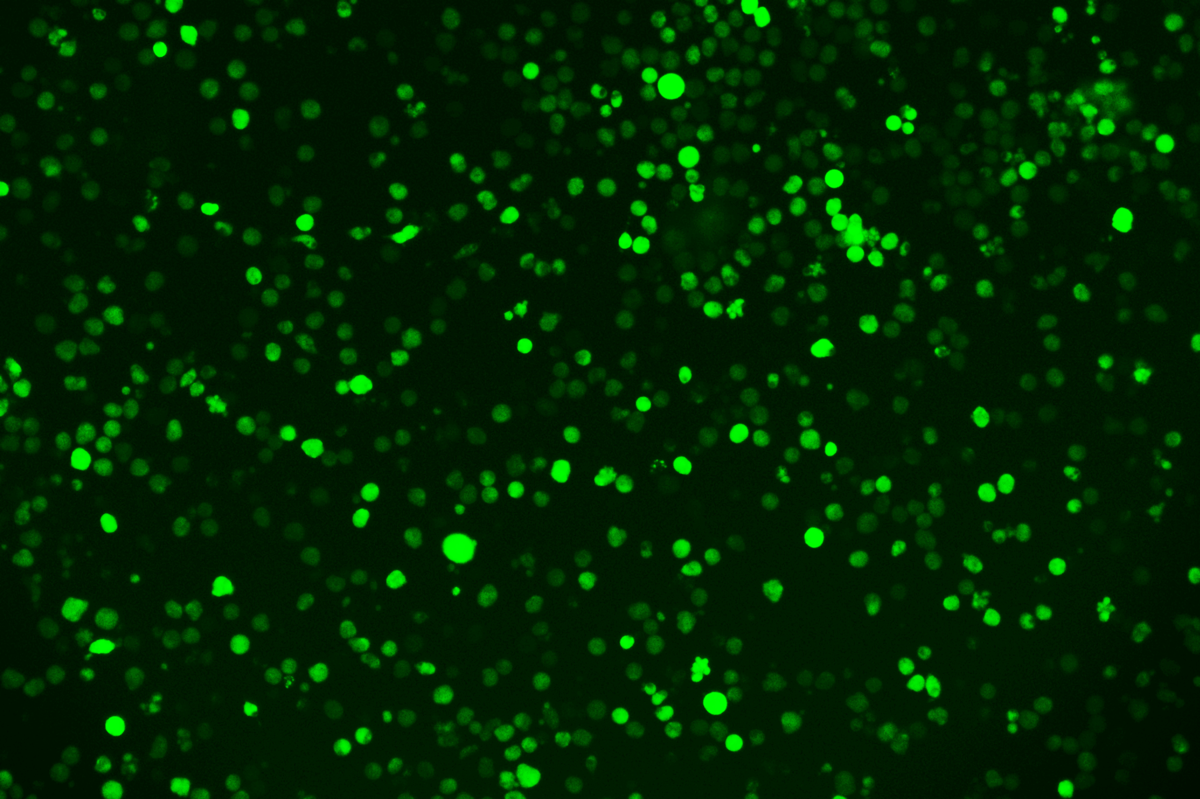
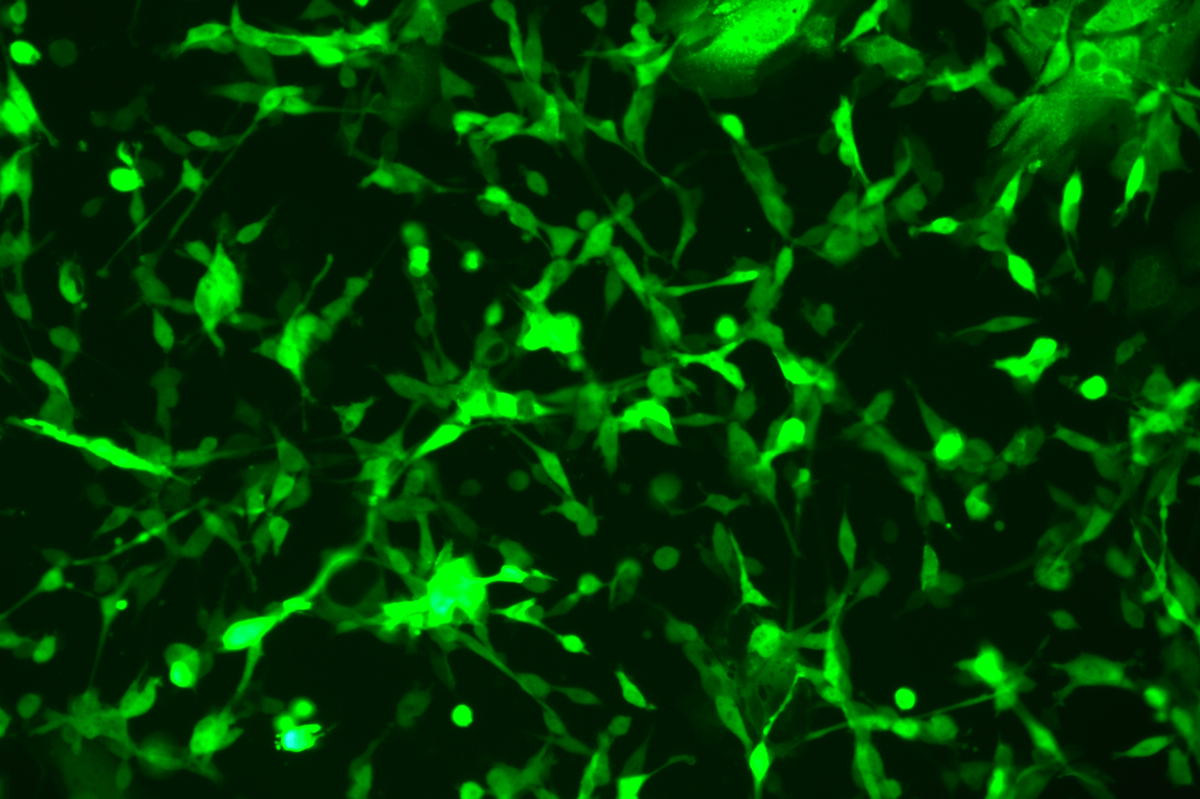
SNT-8-CopGFP Cell line
Cat.No.:
EDC034-CopGFP
Size:
1×10⁶cells
Cell Name:
SNT-8
Introduction
Details Product Information
| Cat.No. | EDC034-CopGFP |
|---|---|
| Product Name | SNT-8-CopGFP Cell line |
| Morphology | Suspension |
| Passage Ratio | 1/3 2-3 days |
| Complete Culture Medium | RPMI-1640+10%FBS+1%P/S |
| Freezing Medium | 50% Basal medium+40%FBS+10%DMSO |
Basic Product Information
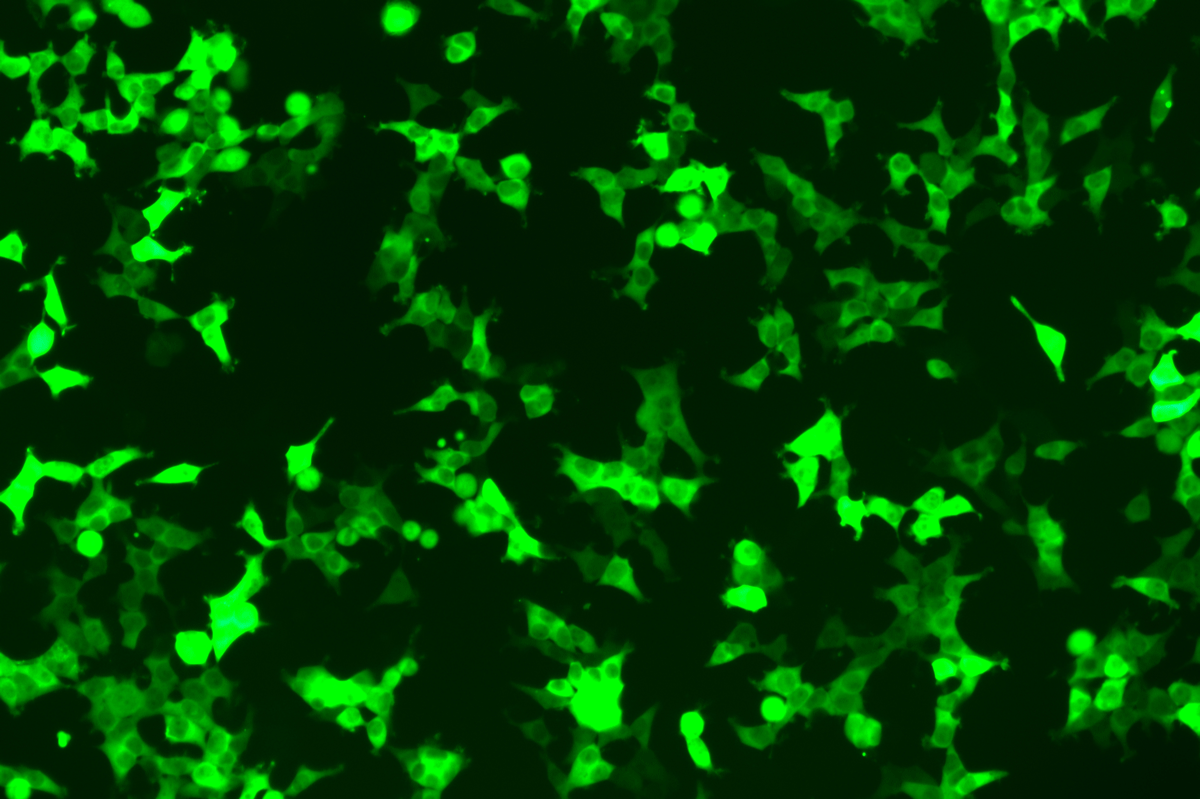
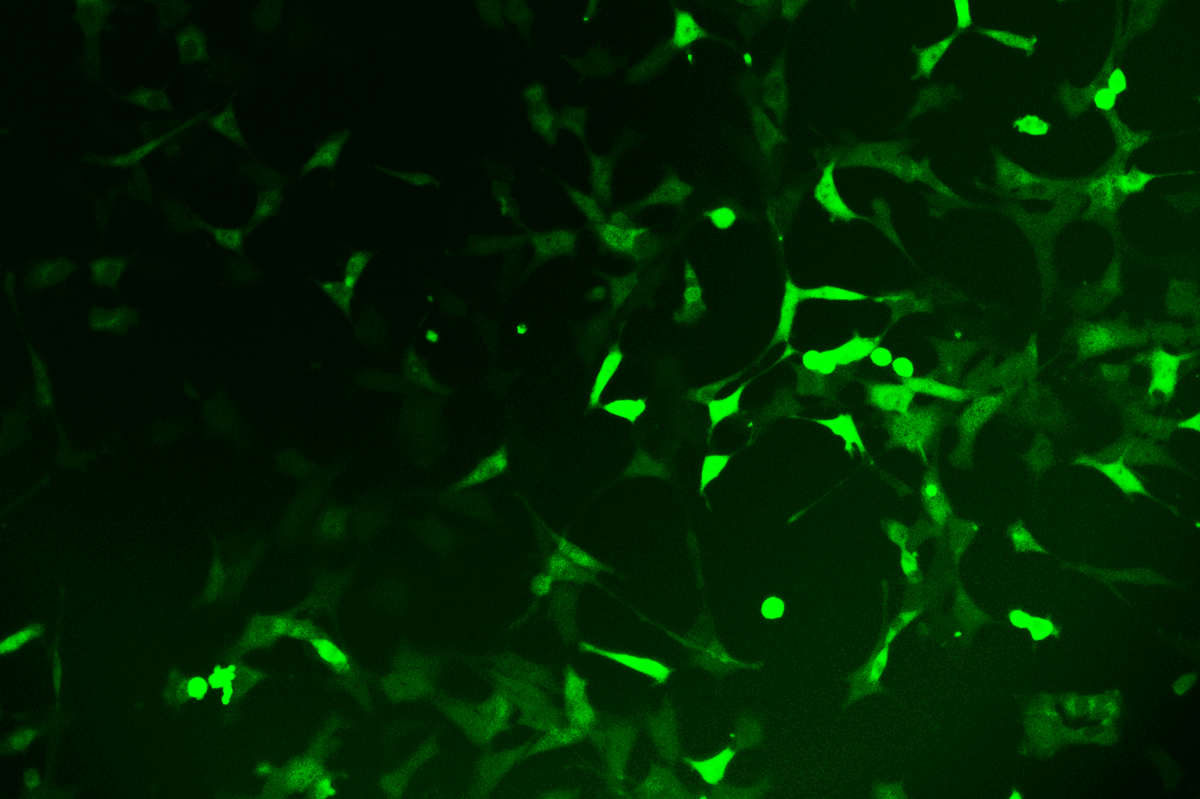
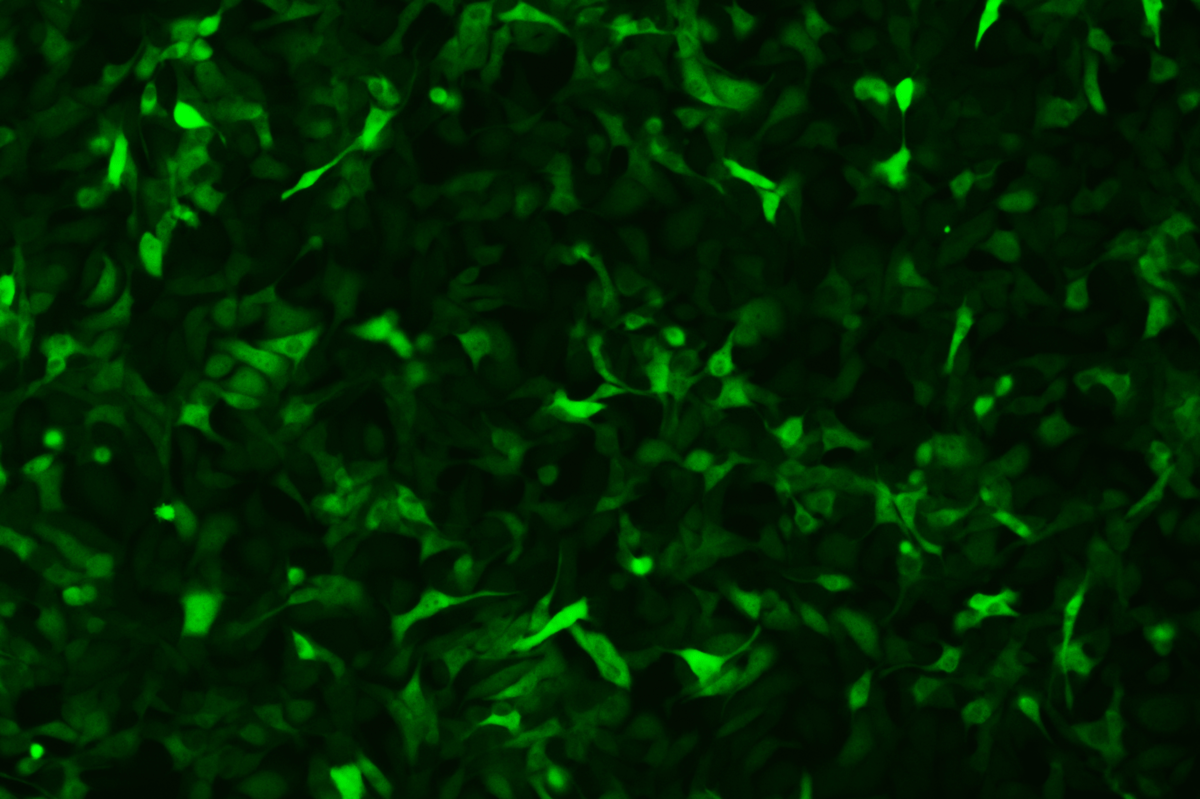
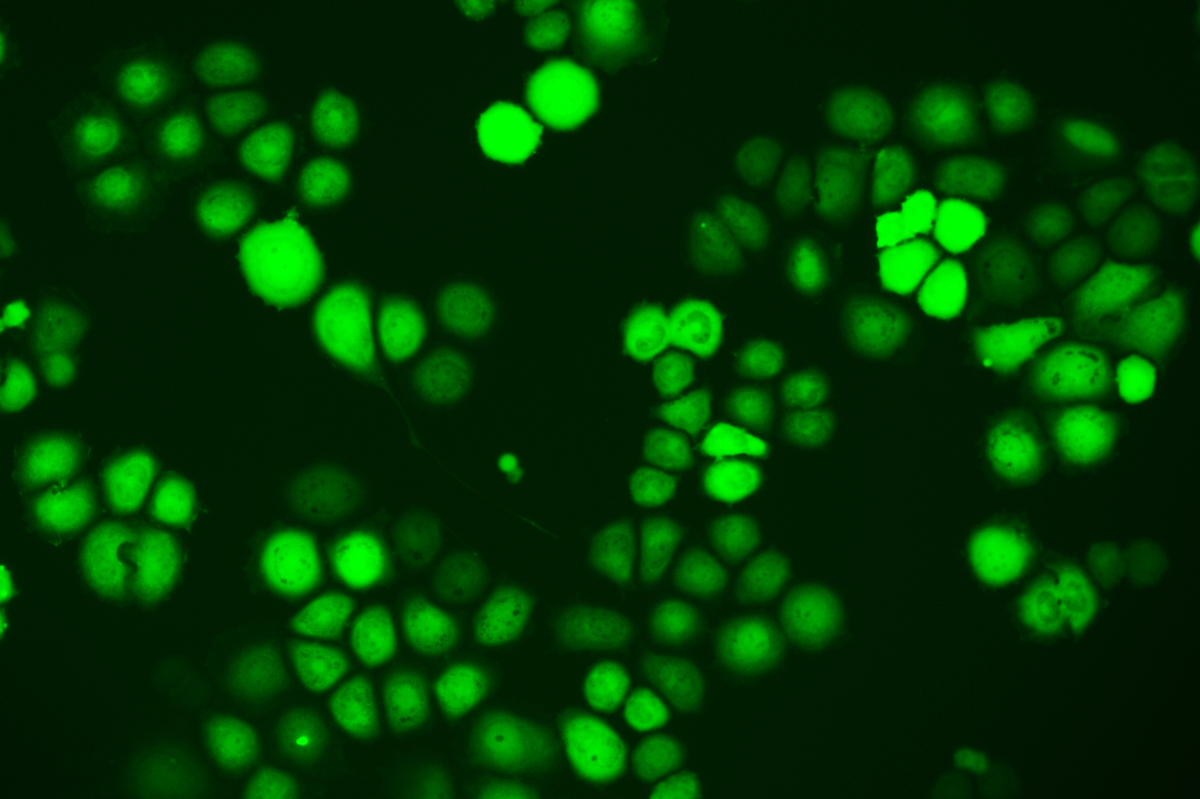
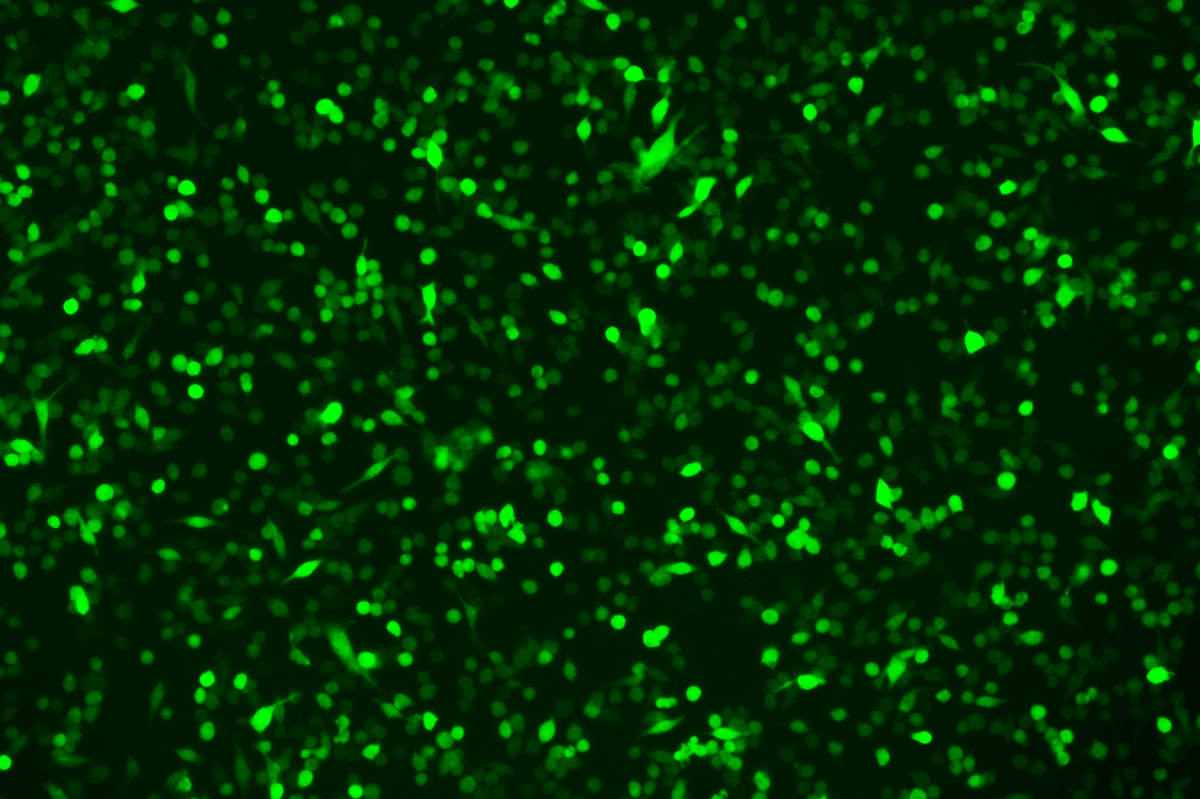
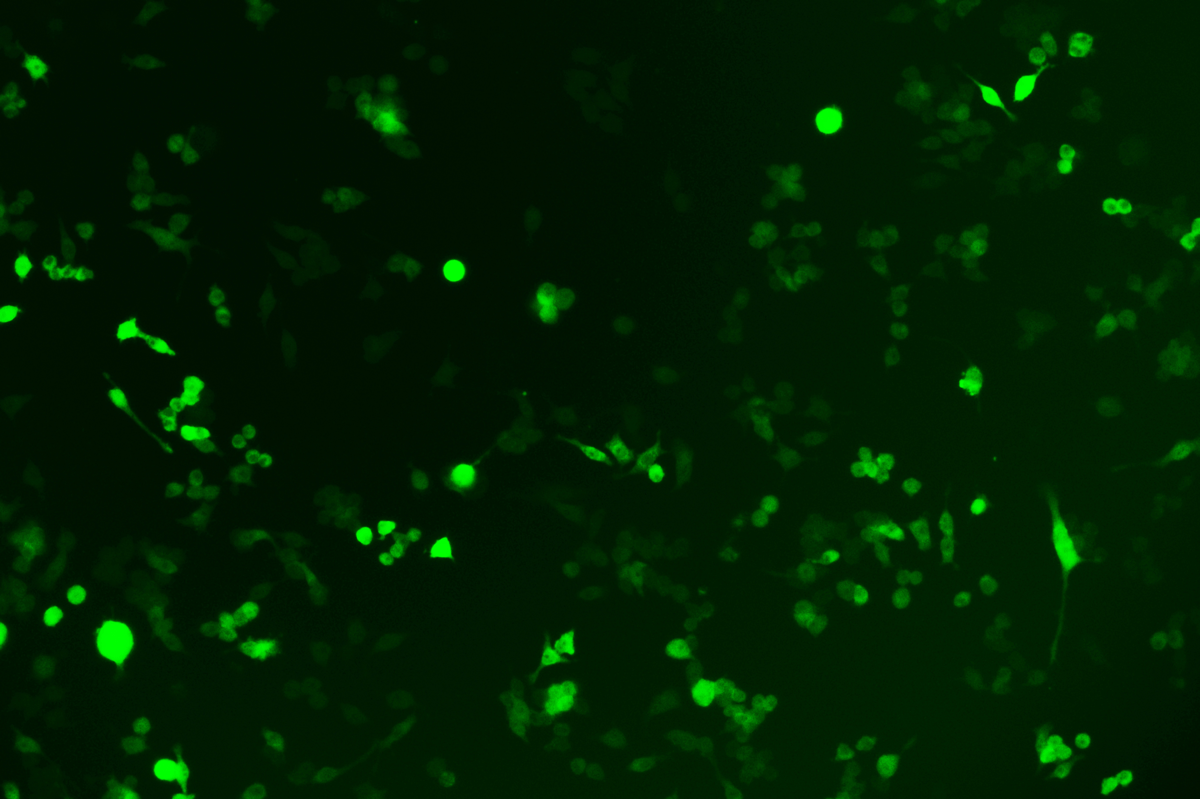
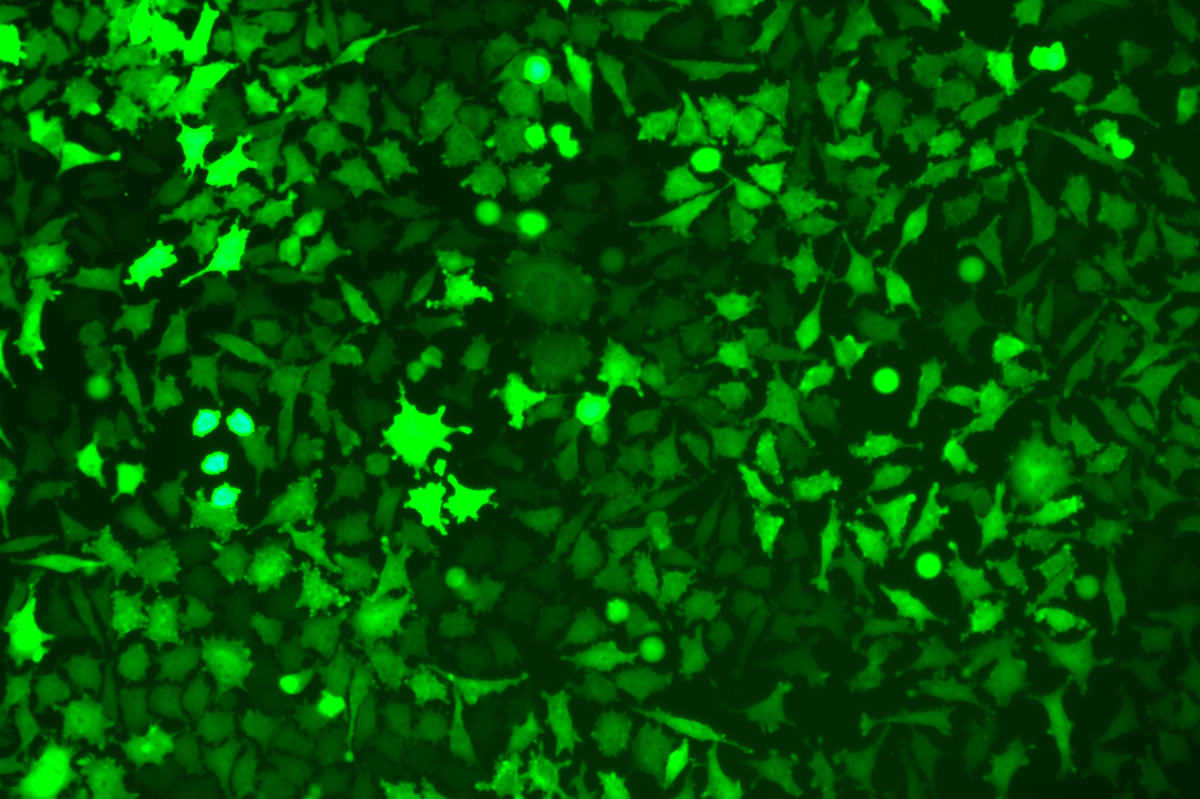
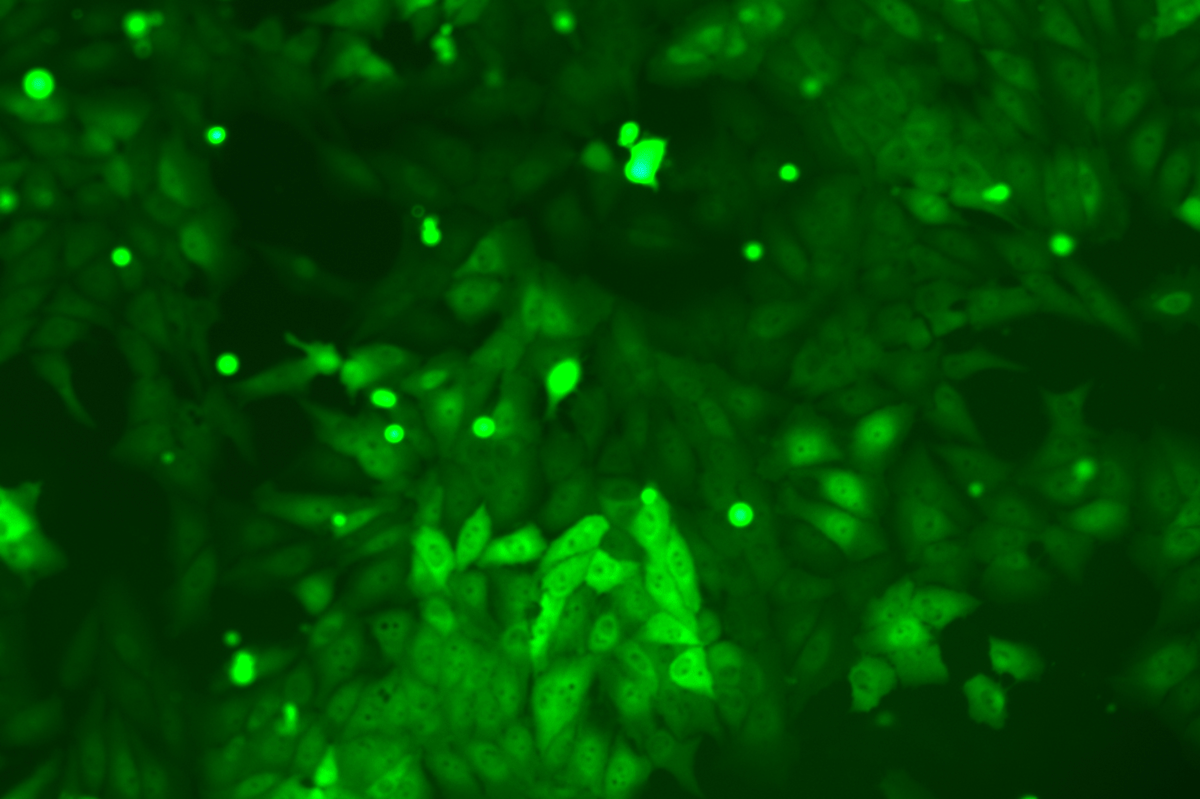
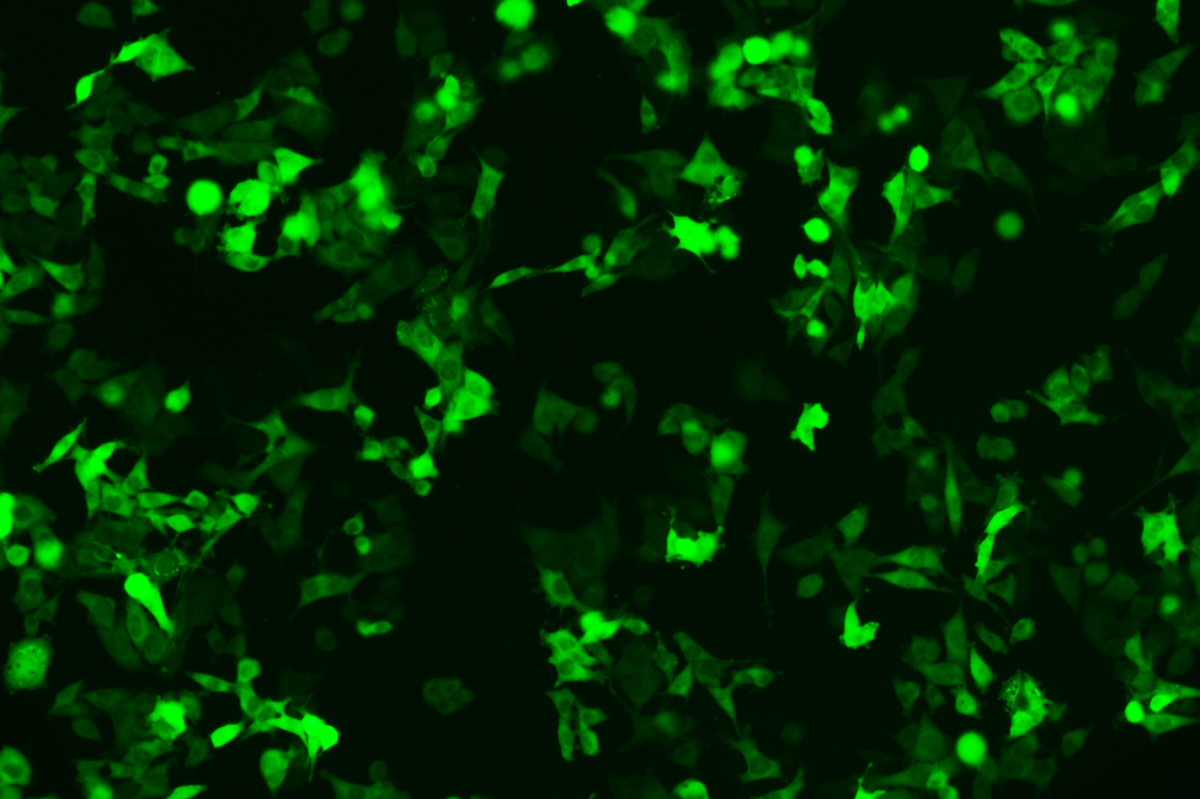
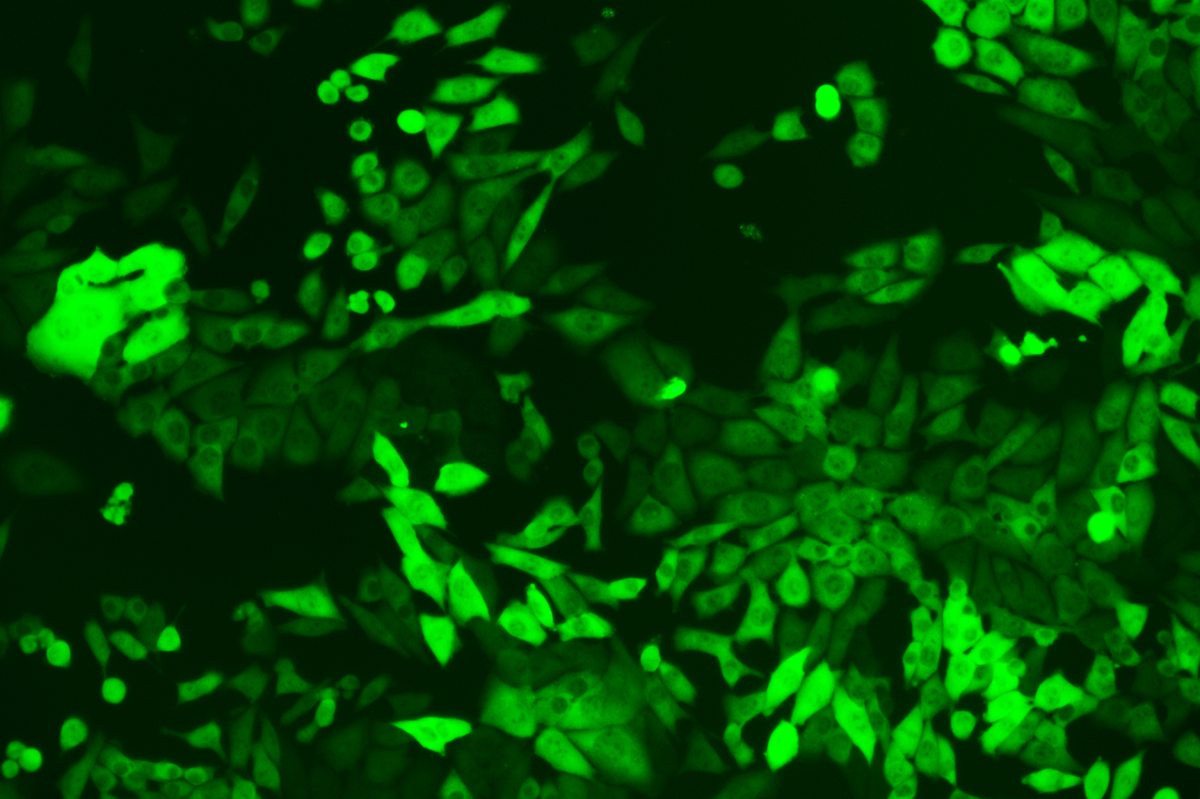
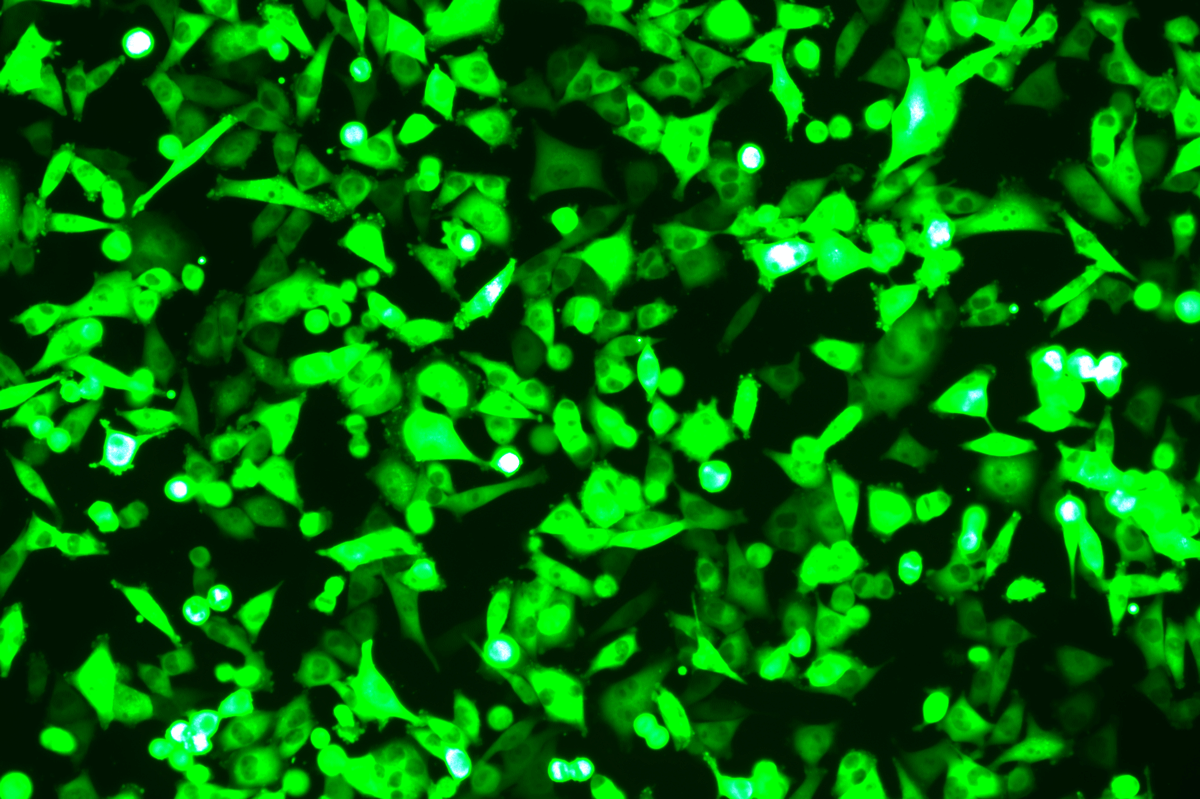
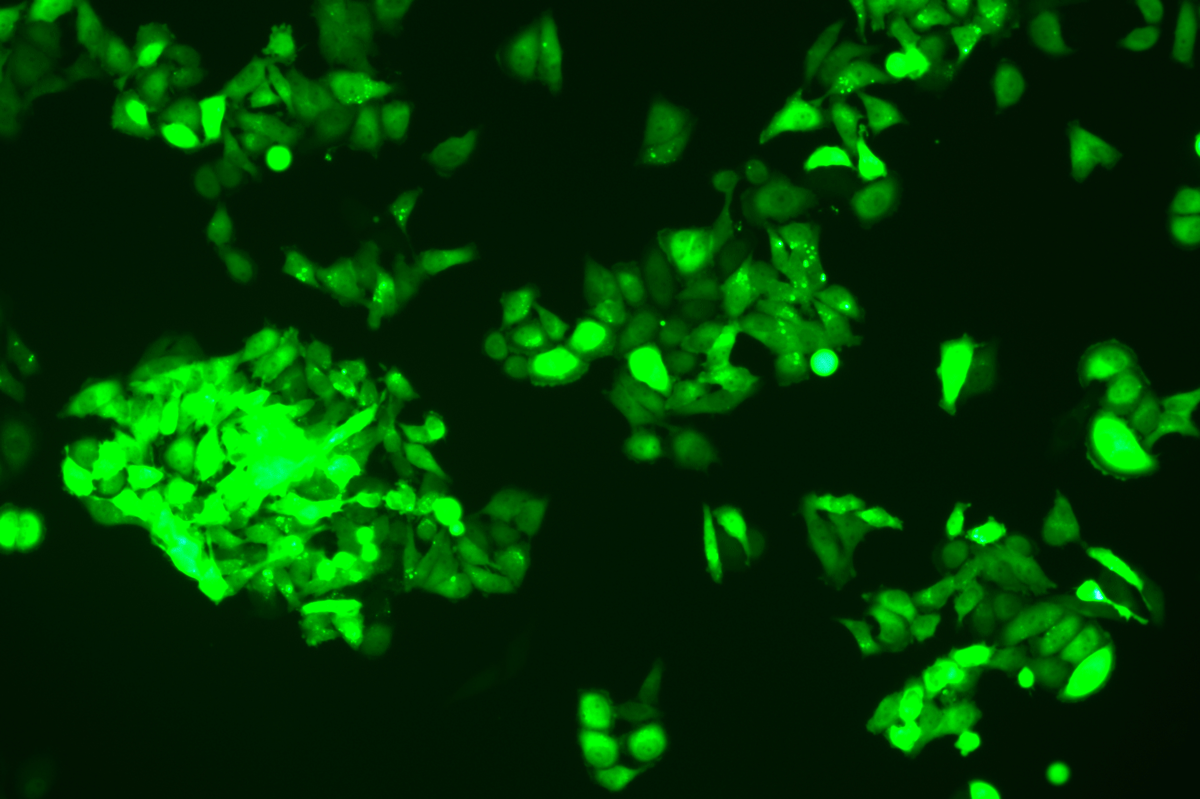
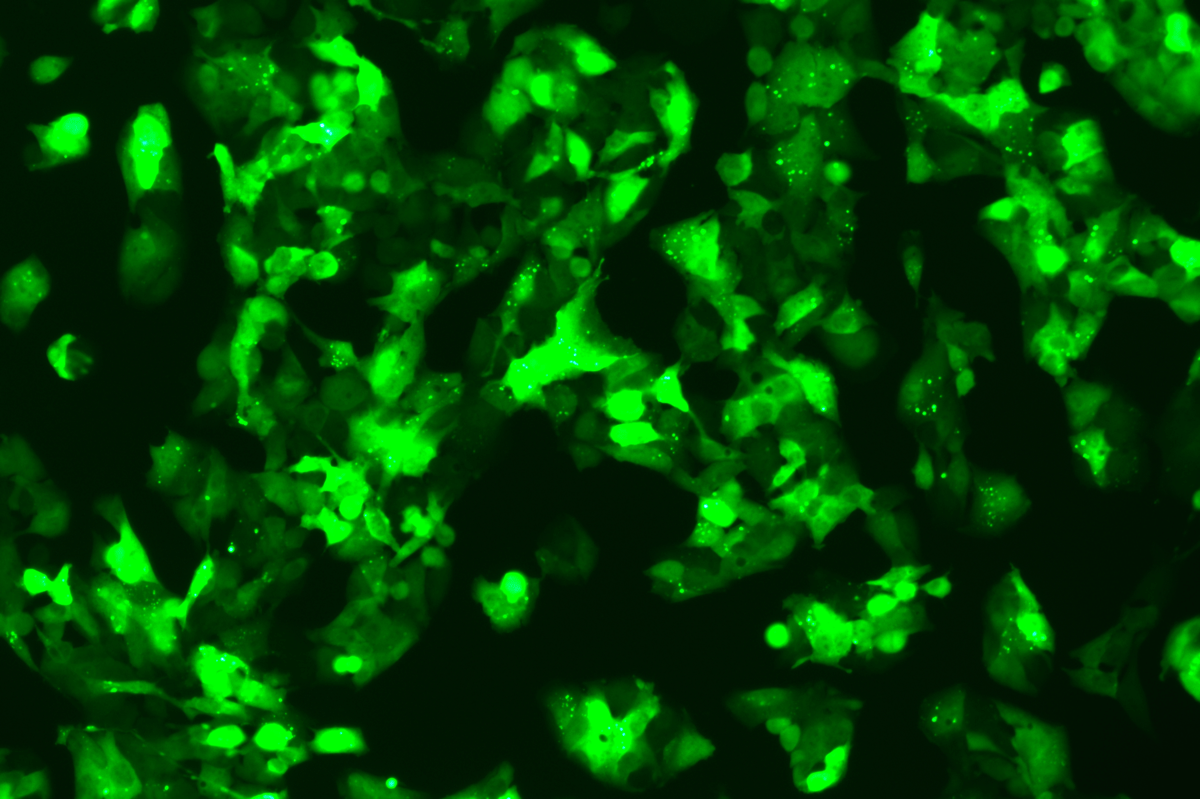
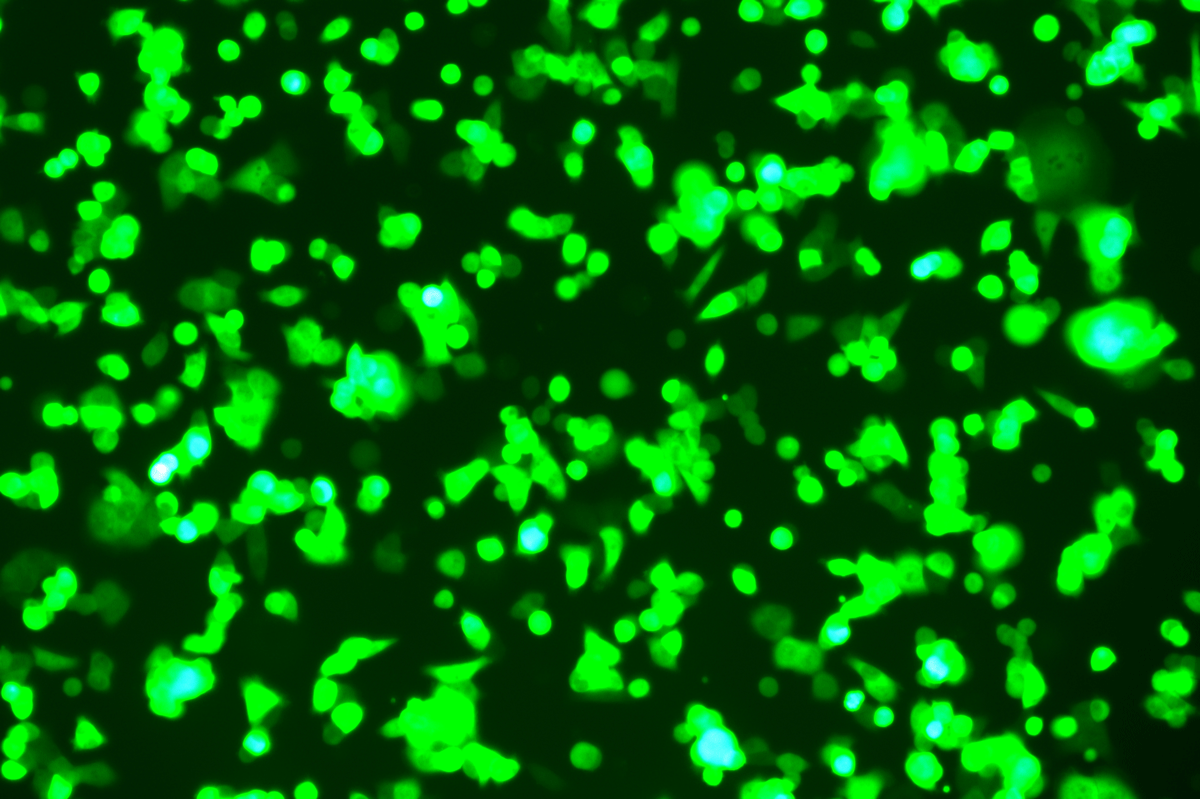
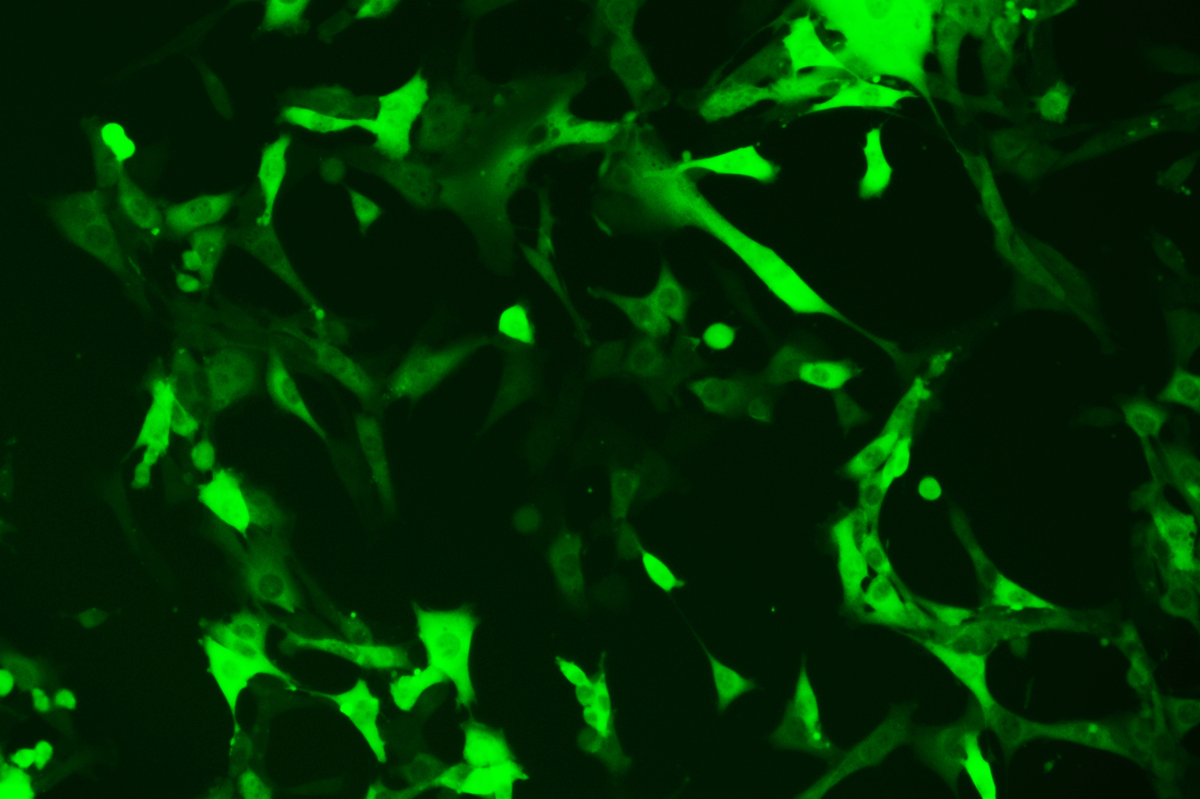
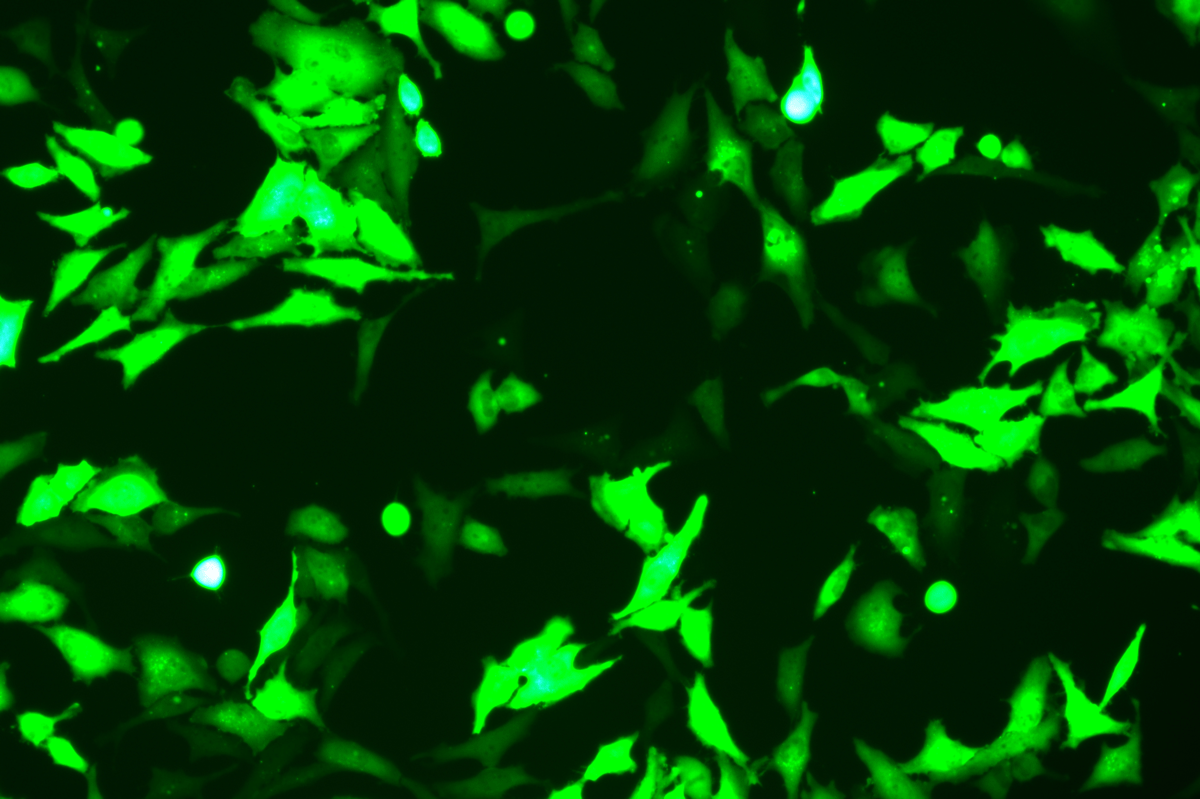
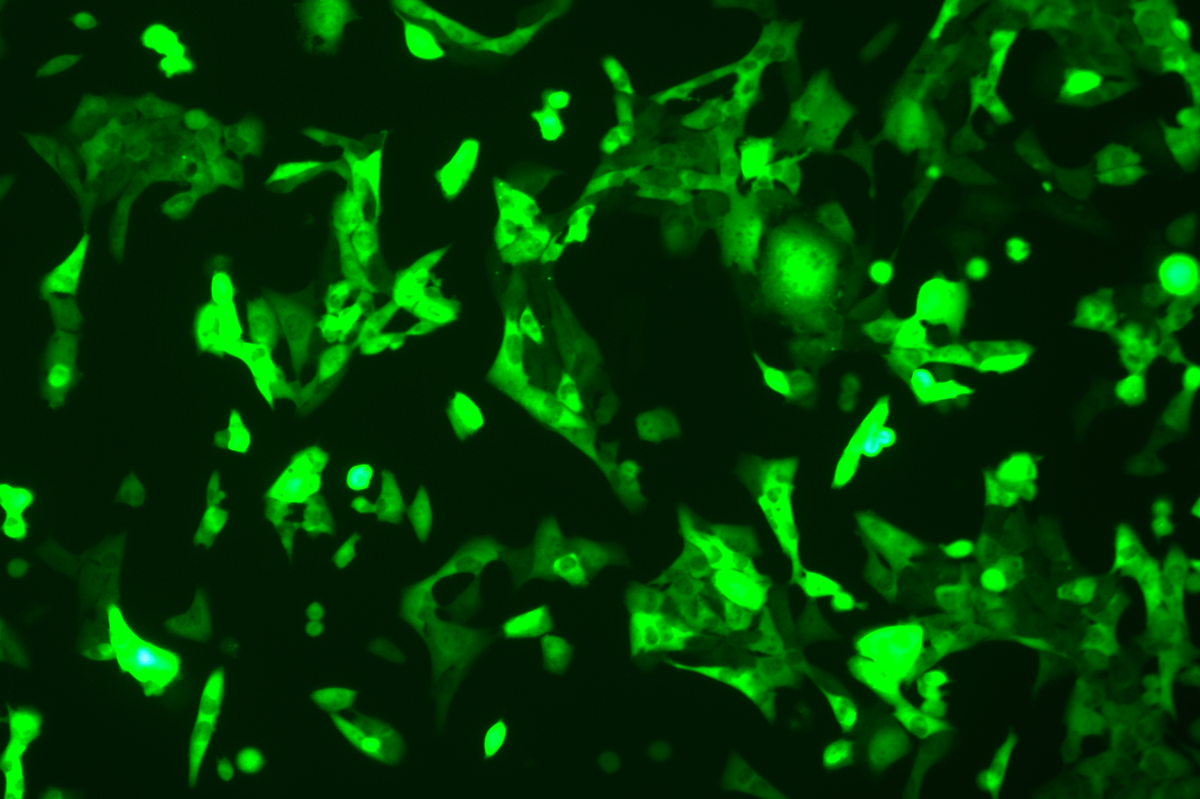
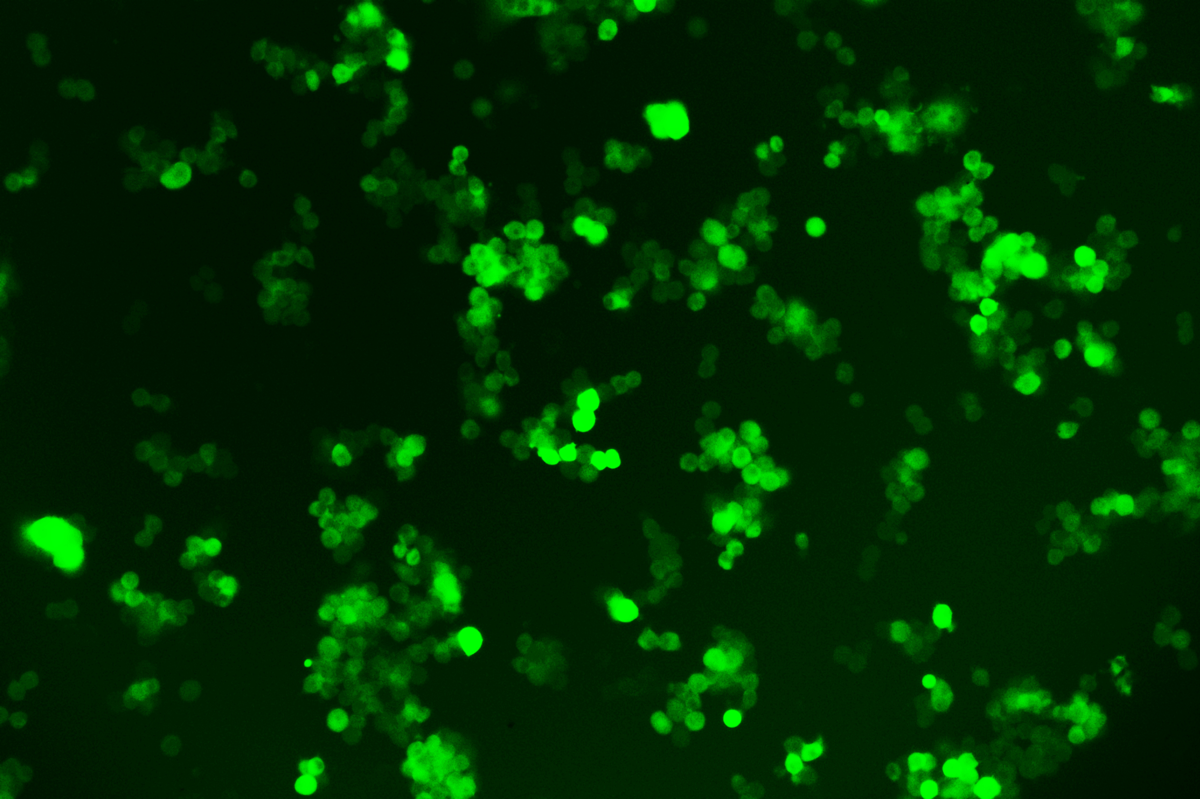
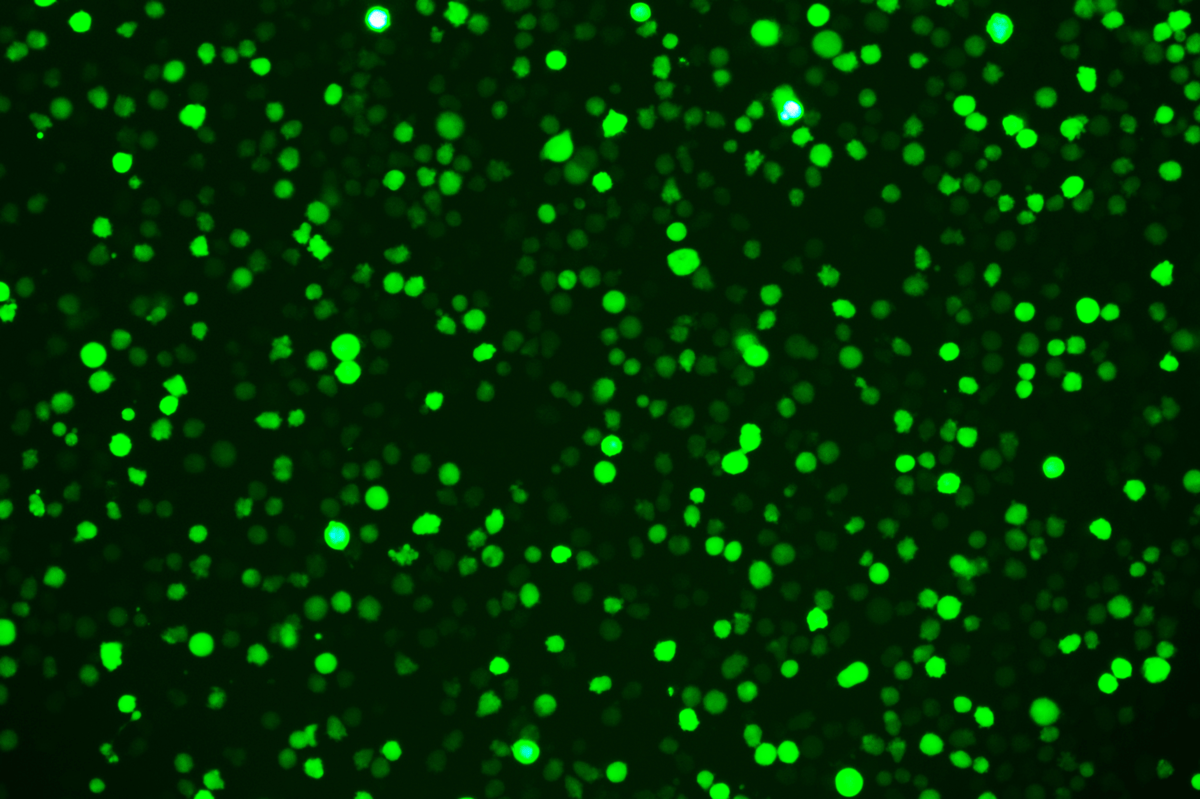
GFP (Green Fluorescent Protein) is a vital tool for observing molecular dynamics within cells. However, it often encounters challenges such as insufficient brightness and limited stability in expression patterns. To tackle these issues, EDITGENE has developed the OE-Booster cis-regulatory element, which enables the production of high-brightness and high-stability CopGFP-tagged cell lines. These cell lines are versatile for use in multiplex fluorescence labeling experiments, enhancing the accuracy of intracellular dynamic observations and improving molecular tracking to reveal complex biological processes.
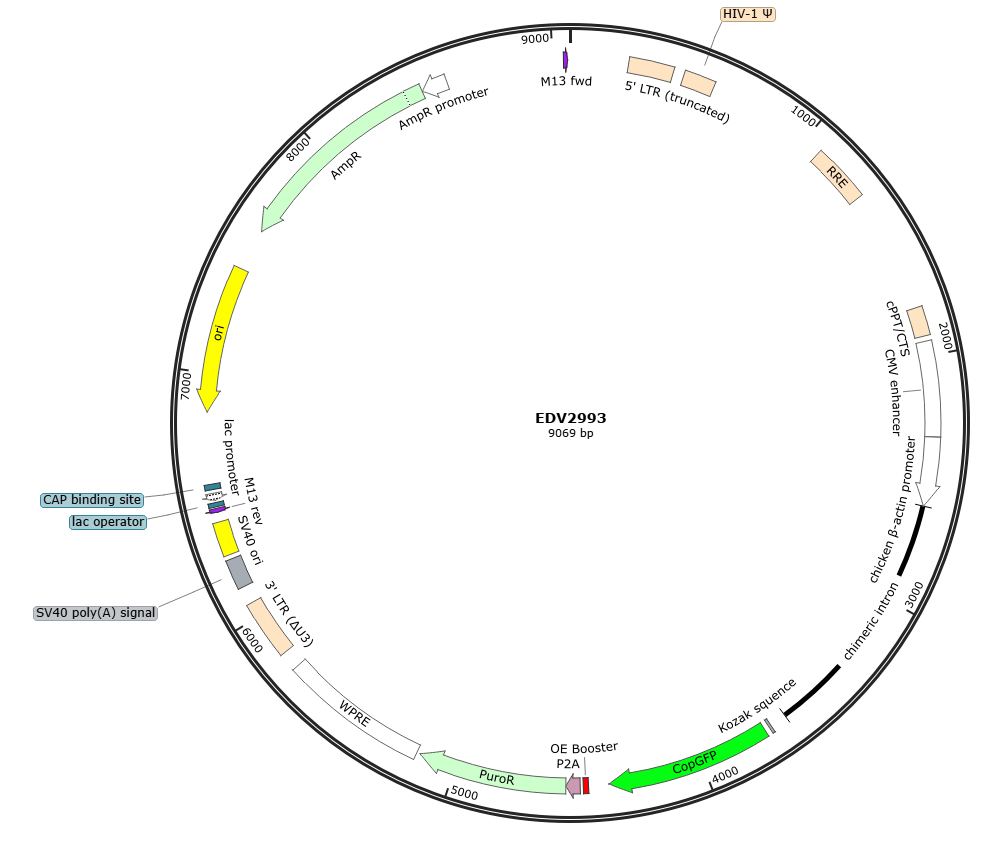
Plasmid Map
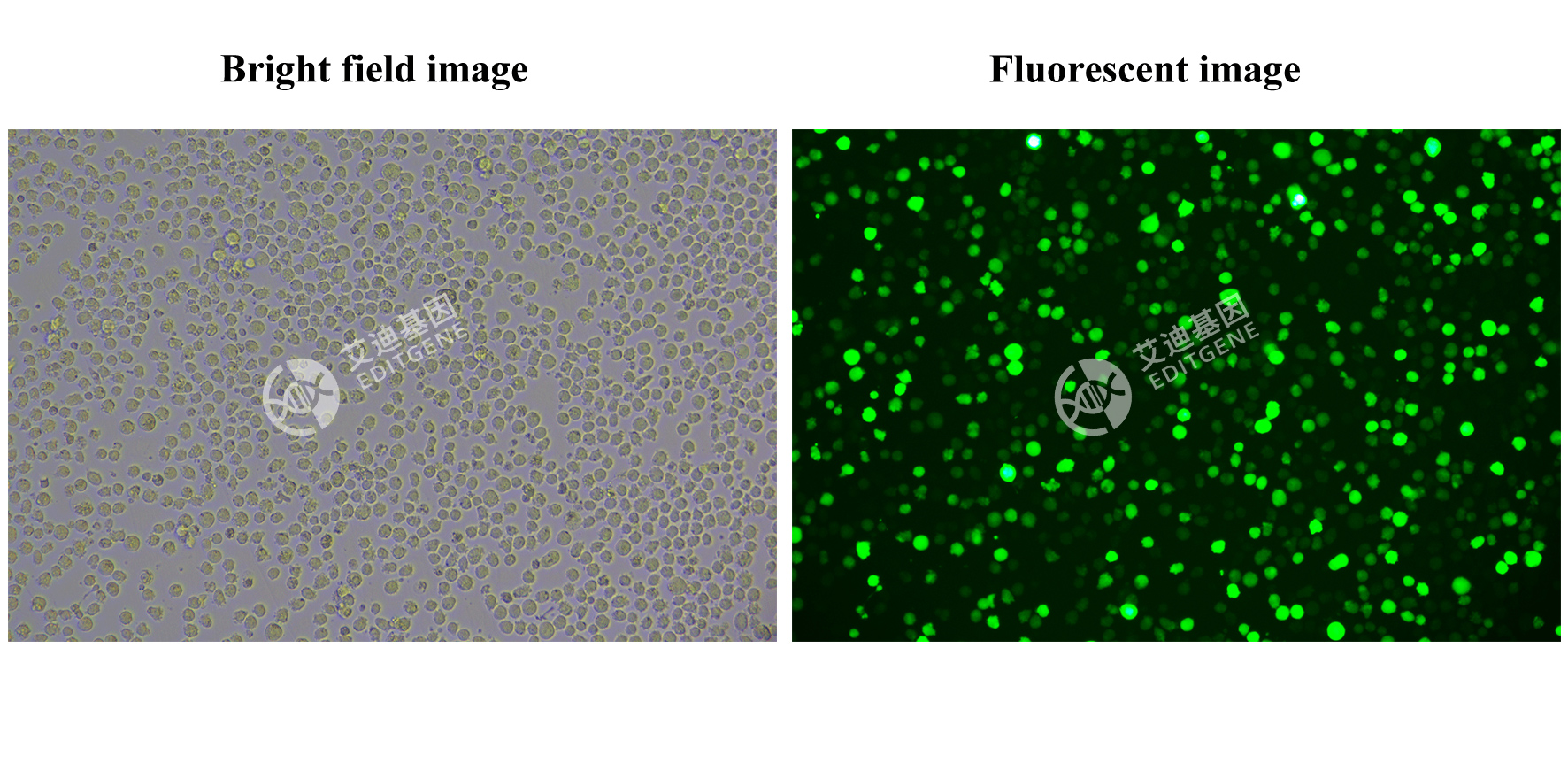
Verification Images
Cell recovery
Note: After receiving the cells, please store in liquid nitrogen. Take it out 10 minutes before recovering cells and place it at -80 ℃ to allow the liquid nitrogen in the tube to evaporate 1. Preheat the water bath and complete culture medium at 37 ℃; 2. Add 6mL of complete culture medium to a 15mL centrifuge tube; 3. Gently swirl the cryopreservation tube in a 37 ℃ water bath until a small piece of ice remains in the cryopreservation tube, please thaw it within 1 minute, and the cap should not touch water; 4. In the ultra clean bench, before opening the cap, wipe the outside of the cryopreservation tube with 75% alcohol; 5. Transfer all the liquid with a pipette to a centrifuge tube of preheated complete culture medium; 6. Rinse the cryopreservation tube with 1mL of complete culture medium to collect residual cells; 7. Centrifuge the cell suspension at 1100 rpm for 4 minutes (centrifugation speed and time depend on cell type); 8. After centrifugation, check if the supernatant is clear and if there is cell pellet at the bottom; inside the hood, carefully pour out the supernatant, add 1mL of complete culture medium, gently resuspend the cell pellet; 9. Evenly seed cells into a T25 flask or culture container with an equivalent bottom area. Add a sufficient amount of complete culture medium, and the total amount of culture medium in a T25 flask shall not be less than 6mL (the actual size of the flask depends on the number of cells frozen in the cryopreservation tube); 10. Gently mix the cells well and place them in a 37 ℃, 5% CO2, saturated humidity incubator (the culture environment depends on cell type and culture medium); 11. Observe the cell status on next day: (1) If the adherent cells adhere well, change fresh complete culture medium; If the cells are observed to be in a round and bright shape but not adhering to the plate, continue cultivation for 24 hours. Afterwards, based on the growth status of the cells, replace the complete culture medium every 2-3 days, observe the cells, and passage cells when confluence rate >80%. If the cell growth is slow or the confluence rate is low, the frequency of medium change can be reduced; (2) When recovering suspension cells, place them in a relatively small container and use a culture medium containing 20% serum for recovery. If the suspended cells are in good condition, change fresh complete culture medium; If the cell status is poor and appears grayscale, it can be further cultured for 72 hours. Change fresh medium if observe live cells. If no significant changes are observed, please contact us for after-sales service in a timely manner.
Passage method
Continuously replenish fresh medium during culturing, observe the cell morphology, and passage cells when they are in a bright state.
1. Preheat the complete culture medium and PBS to 37 ℃;
2. Transfer all cell suspensions to a 15mL centrifuge tube, rinse the flask with PBS (about 3mL for a T25 flask), and collect residual cells;
3. Centrifuge cell suspensions at 1100 rpm for 4 minutes;
4. Discard the supernatant after centrifugation. Add 1mL of complete culture medium, gently resuspend the cell pellet;
5. Seed cells with a passage ratio of 1:2 or 1:3;
Note: Please adjust the passage ratio based on the actual growth of the cells.
6. Place them in an incubator with 37C, 5% CO2 and saturated humidity (if an culture flask is used, the cap should be loosened before putting them into the incubator to facilitate full Gas exchange, unless a ventilated flask or a breathable cap is used);
7. Observe the cell status on the next day;
8. Cells need to be passaged when the confluency >80%.
Storage
Liquid nitrogen


 Login
Login