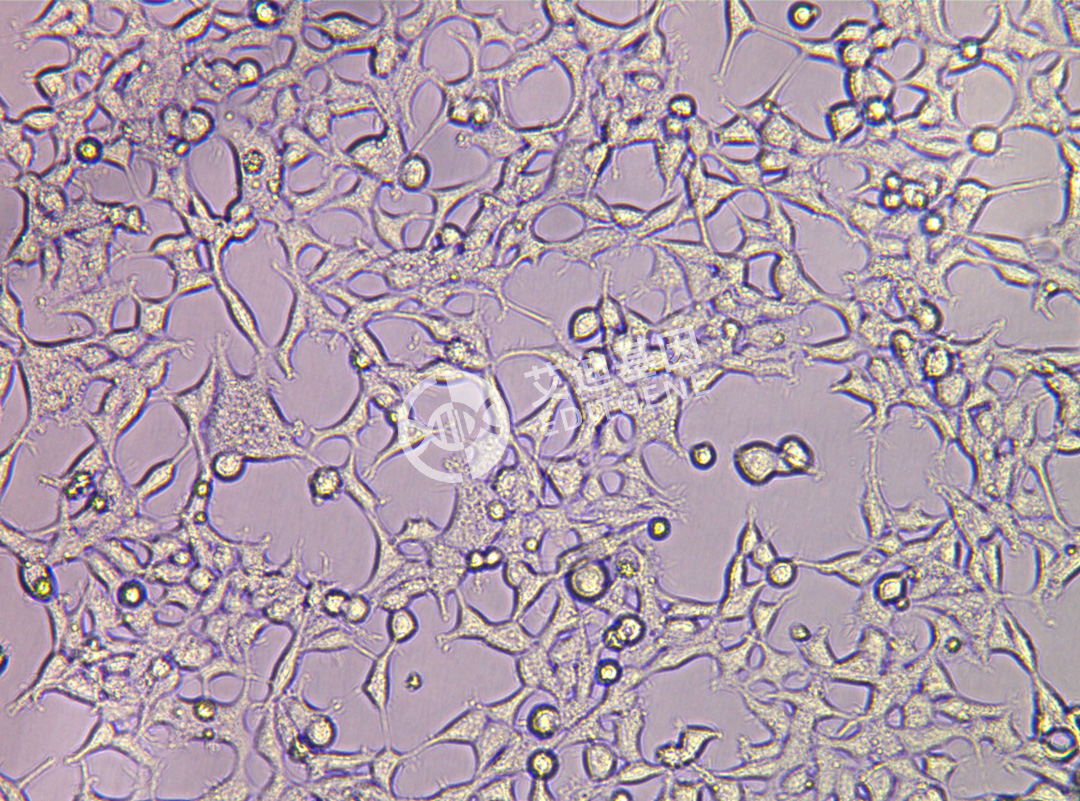
MMP11 knockout cell line (MIA Paca-2)

Cat.No.:
EDJ-KQ58
Gene:
MMP11
Gene ID:
4320
Size:
1×10⁶cells
Cell Form:
semi-adherent semi-suspension
Introduction
Details Product Information
| Cat.No. | EDJ-KQ58 |
|---|---|
| Product Name | MMP11 Knockout Cell Line (MIA Paca-2) |
| Gene | MMP11 |
| Gene Id | 4320 |
| Morphology | semi-adherent semi-suspension |
| Passage Ratio | 1:3-1:8 |
| Complete Culture Medium | 1640+10% FBS |
| Freezing Medium | 95% Complete culture medium + 5% DMSO(ATCC) |
Cell recovery
Note: After receiving the cells, please store in liquid nitrogen. Take it out 10 minutes before recovering cells and place it at -80 ℃ to allow the liquid nitrogen in the tube to evaporate
1. Preheat the water bath and complete culture medium at 37 ℃;
2. Add 6mL of complete culture medium to a 15mL centrifuge tube;
3. Gently swirl the cryopreservation tube in a 37 ℃ water bath until a small piece of ice remains in the cryopreservation tube, please thaw it within 1 minute, and the cap should not touch water;
4. In the ultra clean bench, before opening the cap, wipe the outside of the cryopreservation tube with 75% alcohol;
5. Transfer all the liquid with a pipette to a centrifuge tube of preheated complete culture medium;
6. Rinse the cryopreservation tube with 1mL of complete culture medium to collect residual cells;
7. Centrifuge the cell suspension at 1100 rpm for 4 minutes (centrifugation speed and time depend on cell type);
8. After centrifugation, check if the supernatant is clear and if there is cell pellet at the bottom; inside the hood, carefully pour out the supernatant, add 1mL of complete culture medium, gently resuspend the cell pellet;
9. Evenly seed cells into a T25 flask or culture container with an equivalent bottom area. Add a sufficient amount of complete culture medium, and the total amount of culture medium in a T25 flask shall not be less than 6mL (the actual size of the flask depends on the number of cells frozen in the cryopreservation tube);
10. Gently mix the cells well and place them in a 37 ℃, 5% CO2, saturated humidity incubator (the culture environment depends on cell type and culture medium);
11. Observe the cell status on next day:
(1) If the adherent cells adhere well, change fresh complete culture medium; If the cells are observed to be in a round and bright shape but not adhering to the plate, continue cultivation for 24 hours. Afterwards, based on the growth status of the cells, replace the complete culture medium every 2-3 days, observe the cells, and passage cells when confluence rate >80%. If the cell growth is slow or the confluence rate is low, the frequency of medium change can be reduced;
(2) When recovering suspension cells, place them in a relatively small container and use a culture medium containing 20% serum for recovery. If the suspended cells are in good condition, change fresh complete culture medium; If the cell status is poor and appears grayscale, it can be further cultured for 72 hours. Change fresh medium if observe live cells. If no significant changes are observed, please contact us for after-sales service in a timely manner.
Passage method
1. View cultures using an inverted phase contrast microscope to assess the degree of confluency and confirm the absence of bacterial and fungal contaminants. Give the flask a gentle knock first, this may dislodge the cells from the flask and remove the need for a trypsinisation step loss of some cells due to associated washes;
2. Decant medium containing non-adherent cells into a sterile centrifuge tube and retain;
3. Wash any remaining attached cells with PBS without Ca2+/Mg2+ using 1-2ml for each 25cm2 of surface area. Retain the washings;
4. Pipette trypsin/EDTA onto the washed cell monolayer using 1ml per 25cm2 of surface area. Rotate flask to cover the monolayer with trypsin. Decant the excess trypsin;
5. Return flask to incubator and leave for 2-10 minutes;
6. Examine the cells using an inverted microscope to ensure that all the cells are detached and floating. The side of the flasks may be gently tapped to release any remaining attached cells;
7. Transfer the cells into the centrifuge tube containing the retained spent medium and cells;
8. Centrifuge the entire cell suspension at 150 x g for 5 minutes;
9. Remove the supernatant and re-suspend the cell pellet in a small volume (10-20ml) of fresh
culture medium. Count the cells;
10. Pipette the required number of cells into a new labelled flask and dilute to the required volume
using fresh medium;
11. Repeat this process every 2-3 days as necessary.
Storage
Liquid nitrogen
Related Product
-
Gene: EFNA5
Gene ID: 1946
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: DTX3
Gene ID: 196403
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: IL1A
Gene ID: 3552
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: AOAH
Gene ID: 313
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: WNT11
Gene ID: 7481
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: MAP1LC3C
Gene ID: 440738
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: CDS1
Gene ID: 1040
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: KDM6A
Gene ID: 7403
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: TYMP
Gene ID: 1890
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: ACKR3
Gene ID: 57007
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: RAD23A
Gene ID: 5886
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: HNRNPA3
Gene ID: 220988
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: CHRNA3
Gene ID: 1136
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: GPT
Gene ID: 2875
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: PHLDA2
Gene ID: 7262
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: ACTN3
Gene ID: 89
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: CEACAM8
Gene ID: 1088
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: ELAVL2
Gene ID: 1993
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: GPR31
Gene ID: 2853
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: ANOS1
Gene ID: 3730
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: MYH11
Gene ID: 4629
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: PRKX
Gene ID: 5613
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: SLC22A1
Gene ID: 6580
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: ZNF69
Gene ID: 7620
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: UNC5C
Gene ID: 8633
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: RAB9A
Gene ID: 9367
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: PAN2
Gene ID: 9924
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: FBXW10
Gene ID: 10517
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: KLK8
Gene ID: 11202
Size: 1×10⁶cells
Cell Form: Adherent
-
Gene: ART5
Gene ID: 116969
Size: 1×10⁶cells
Cell Form: Adherent
Subscribe
You can unsubscribe from these communications at any time. For more information on how to unsubscribe, our privacy practices, and how we are committed to protecting and respecting your privacy, please review our Privacy Policy.
By clicking submit below, you consent to allow EDITGENE to store and process the personal information submitted above to provide you the content requested.


 Login
Login