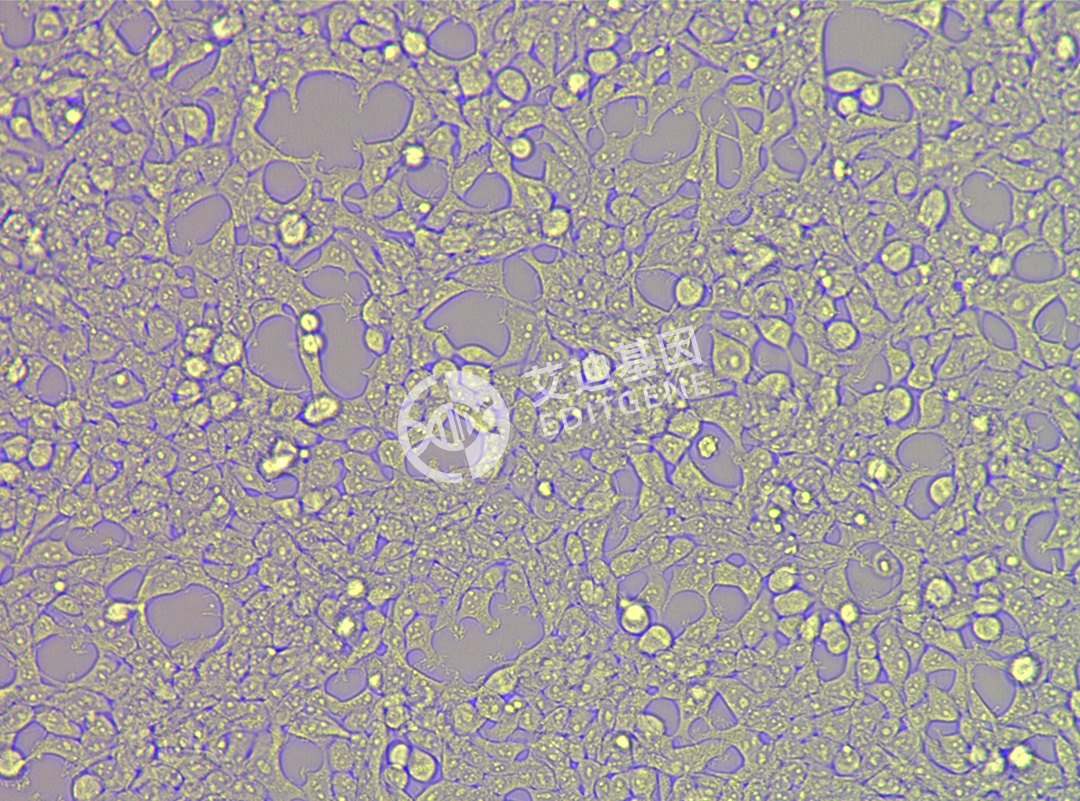
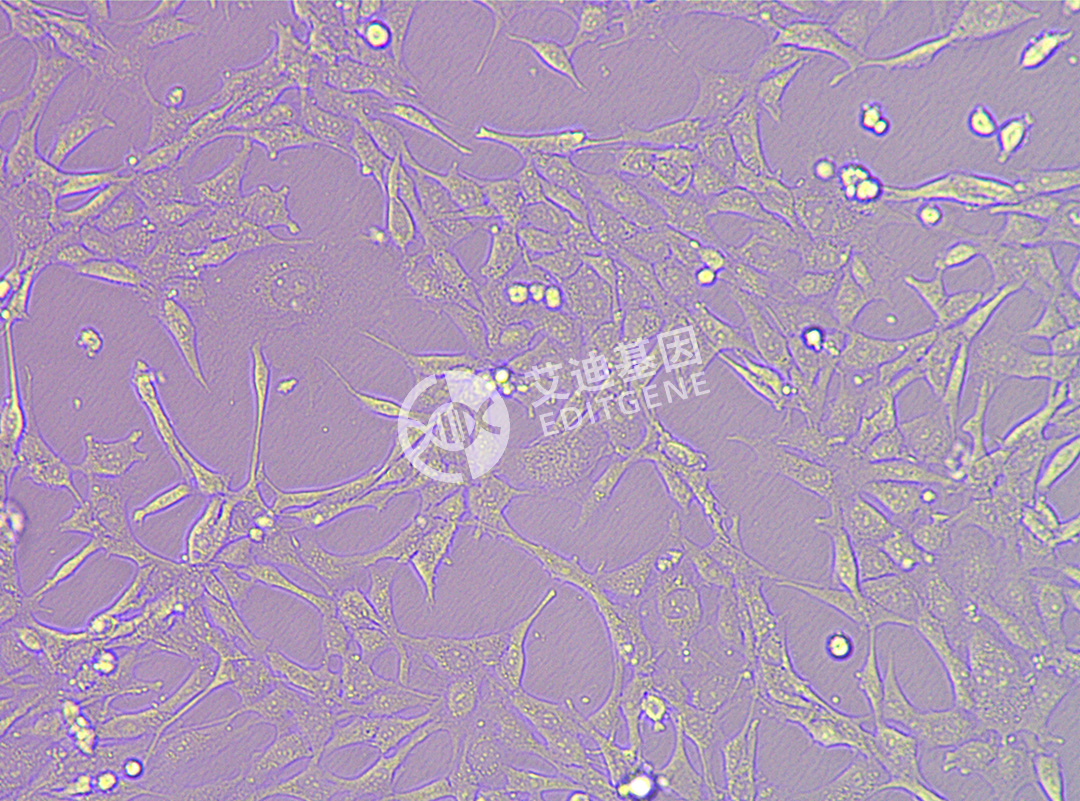
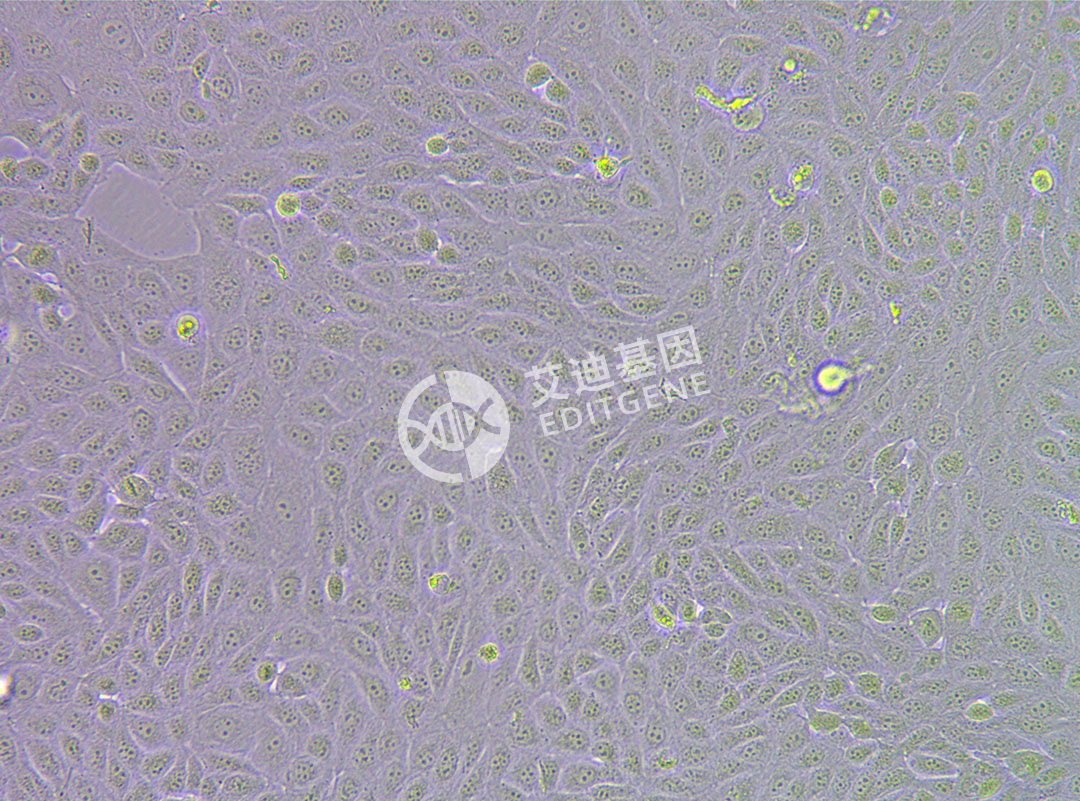
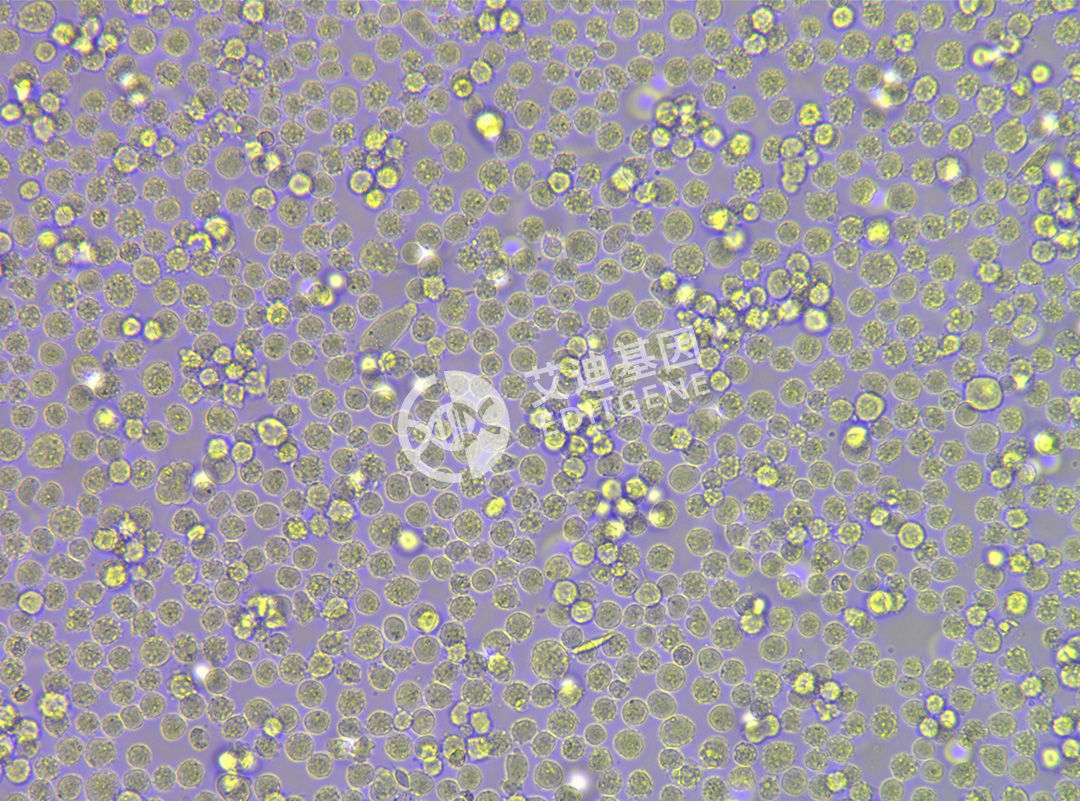
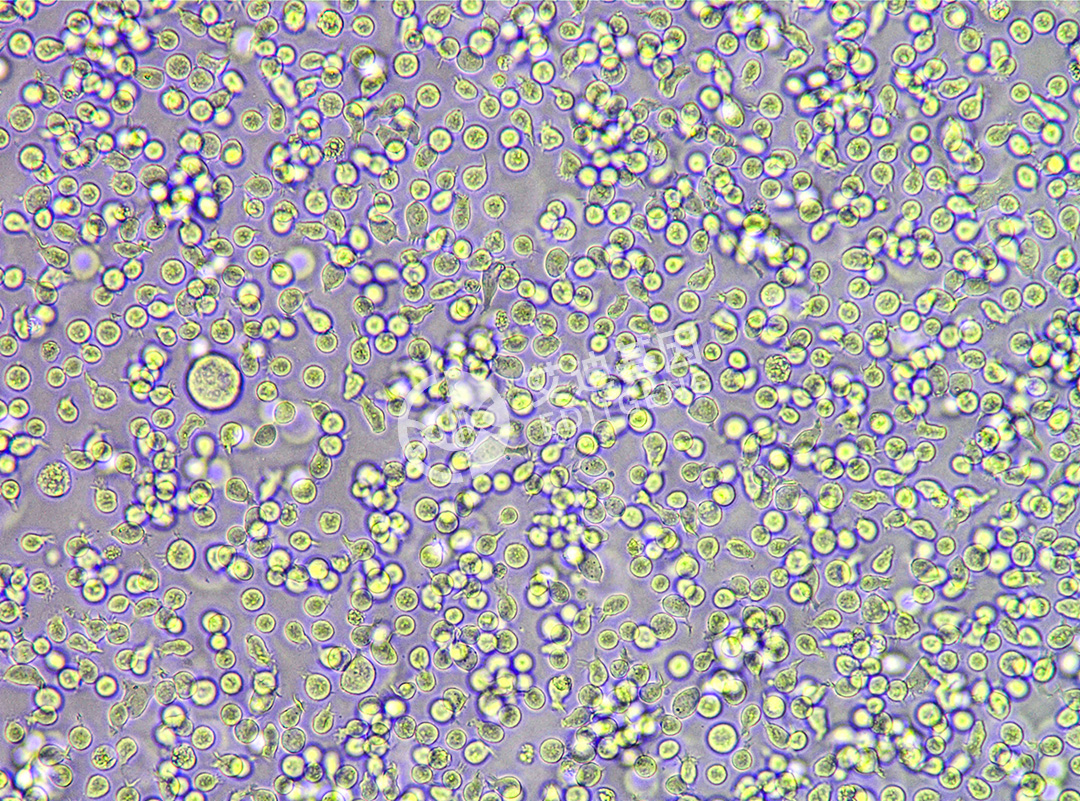
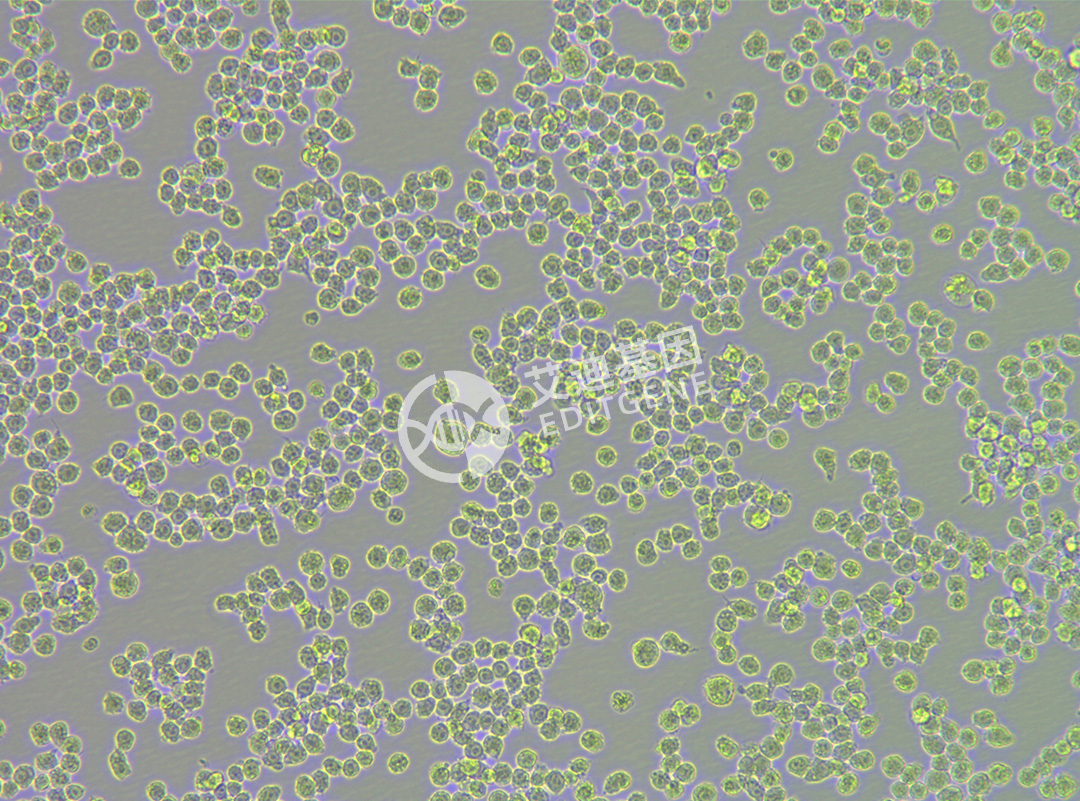
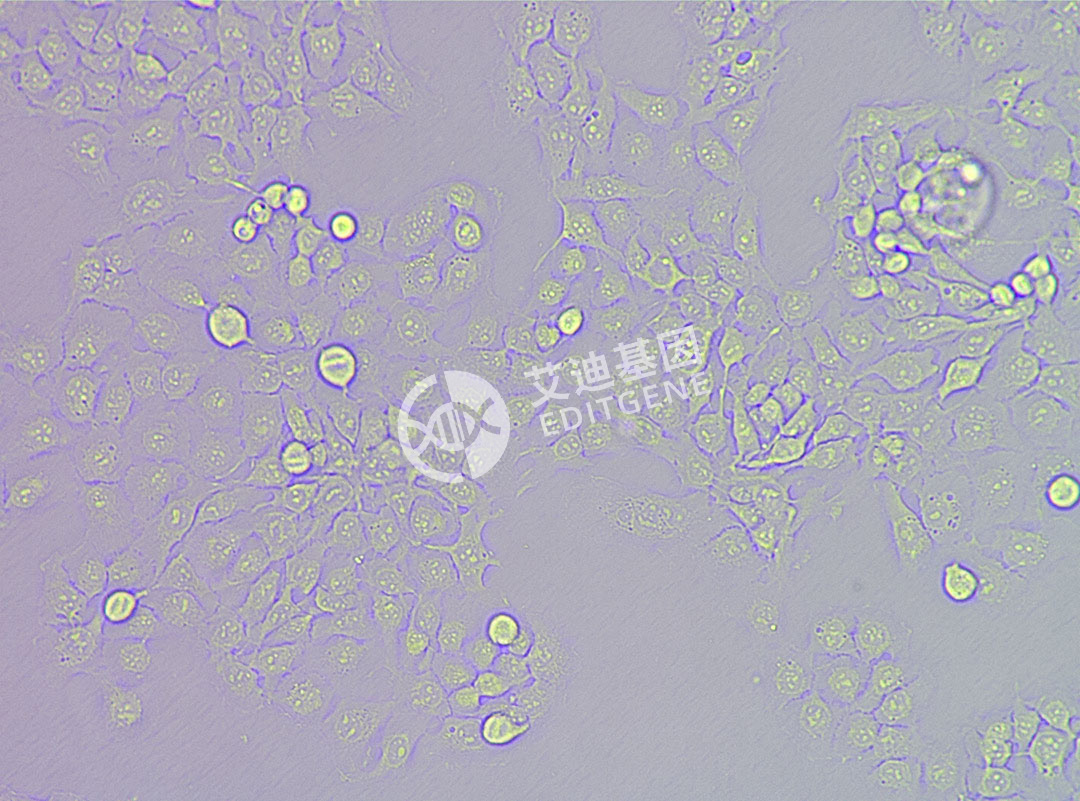
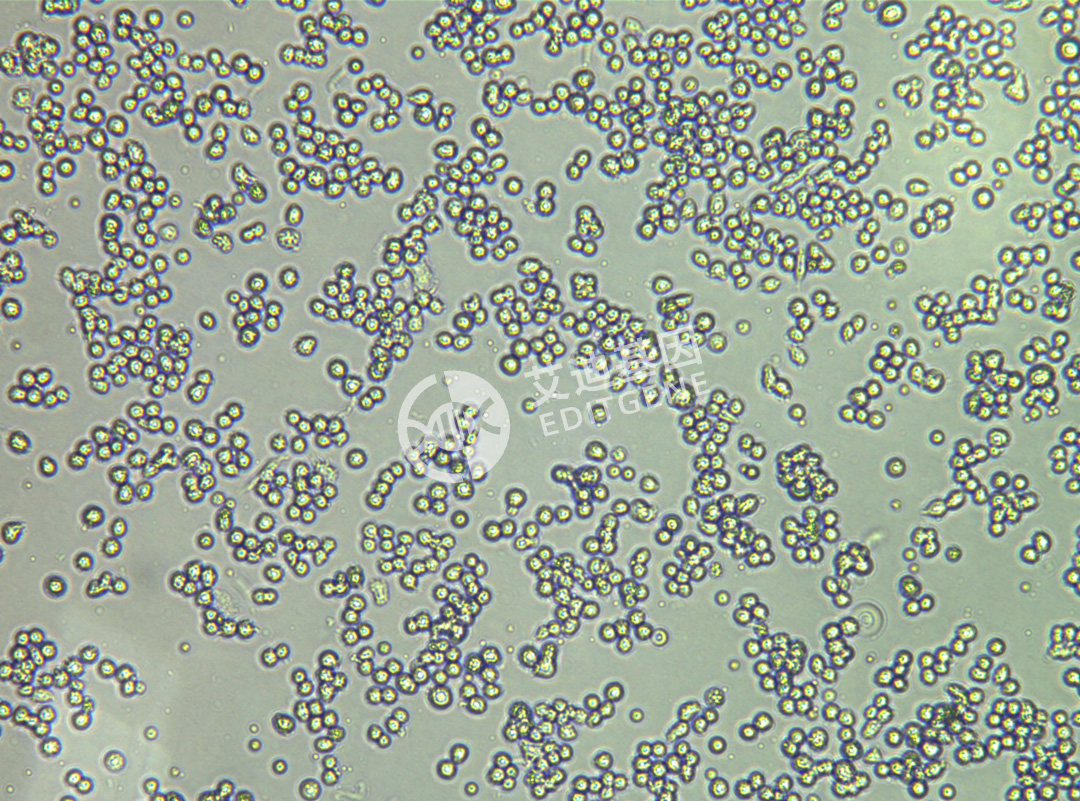
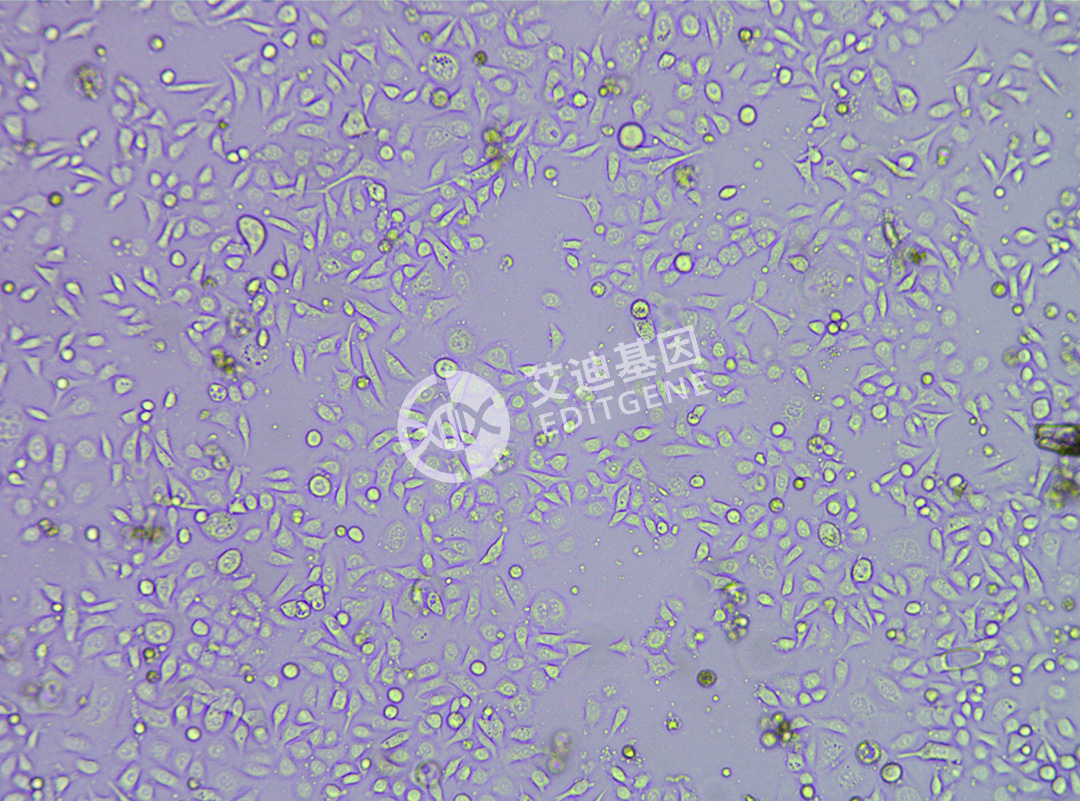
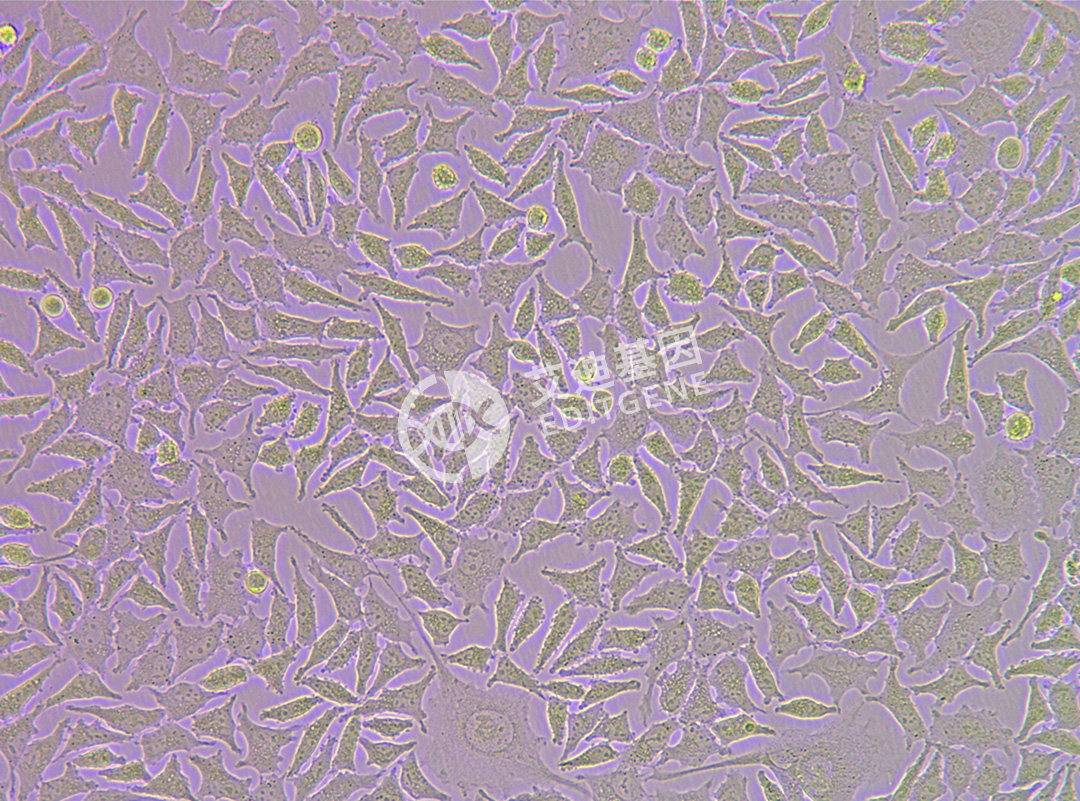
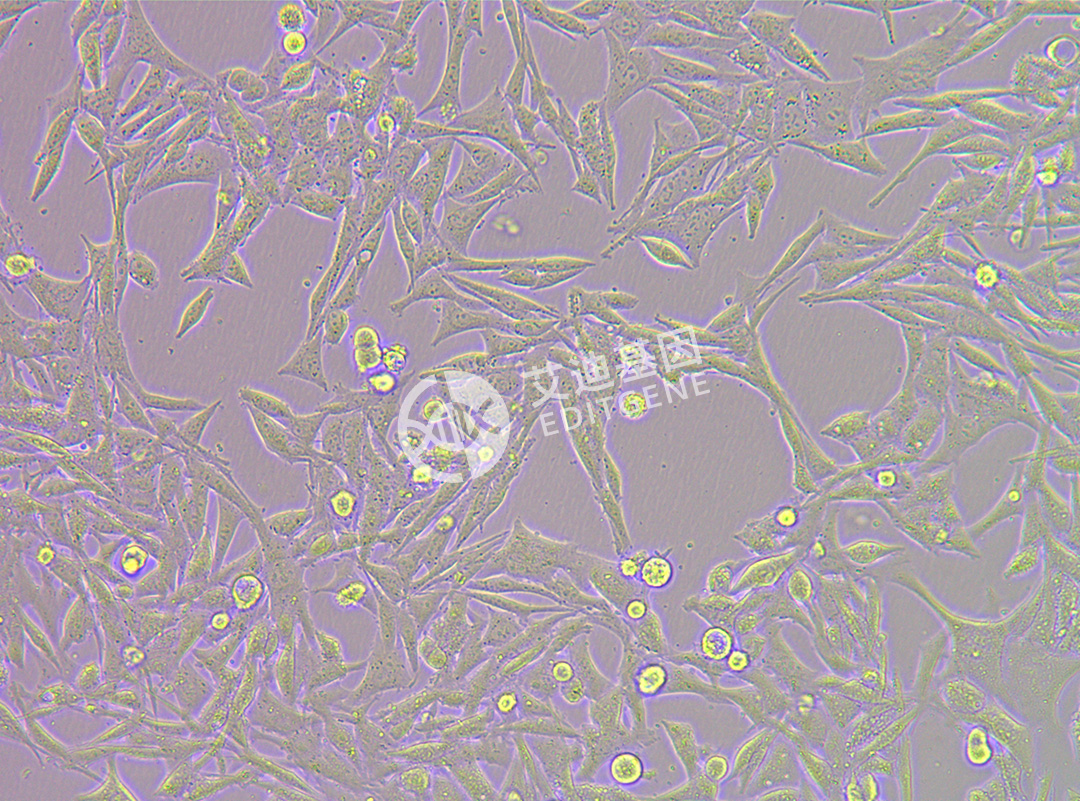
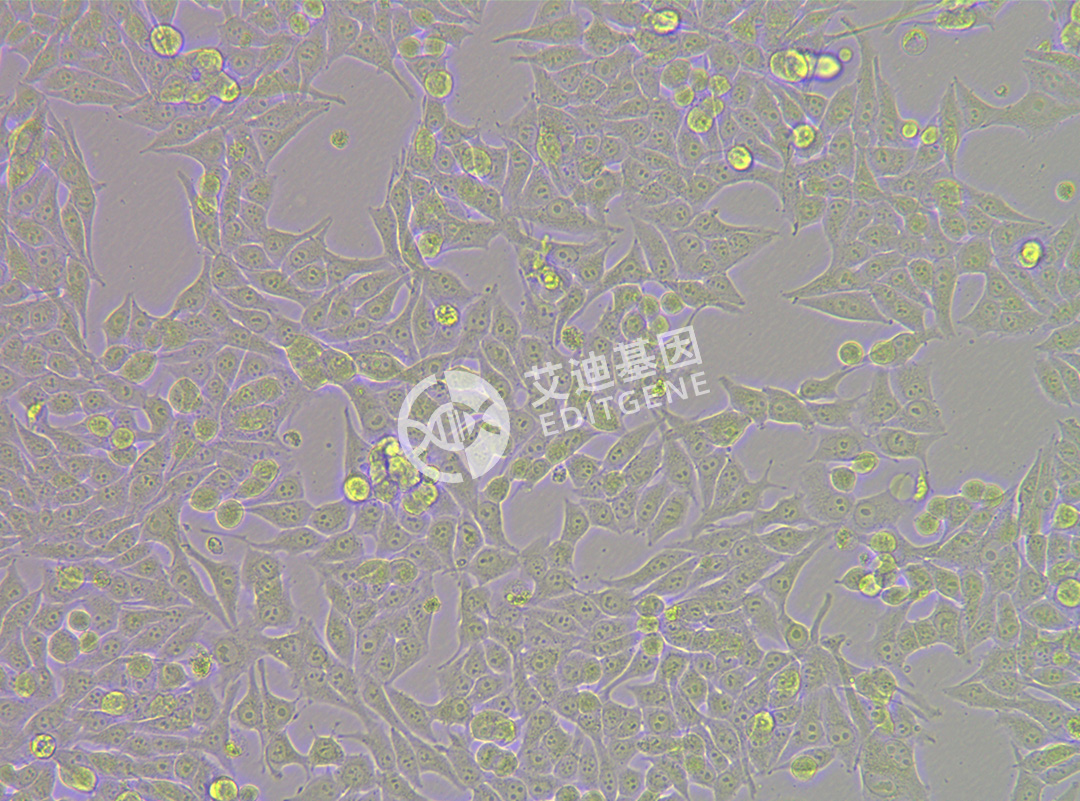
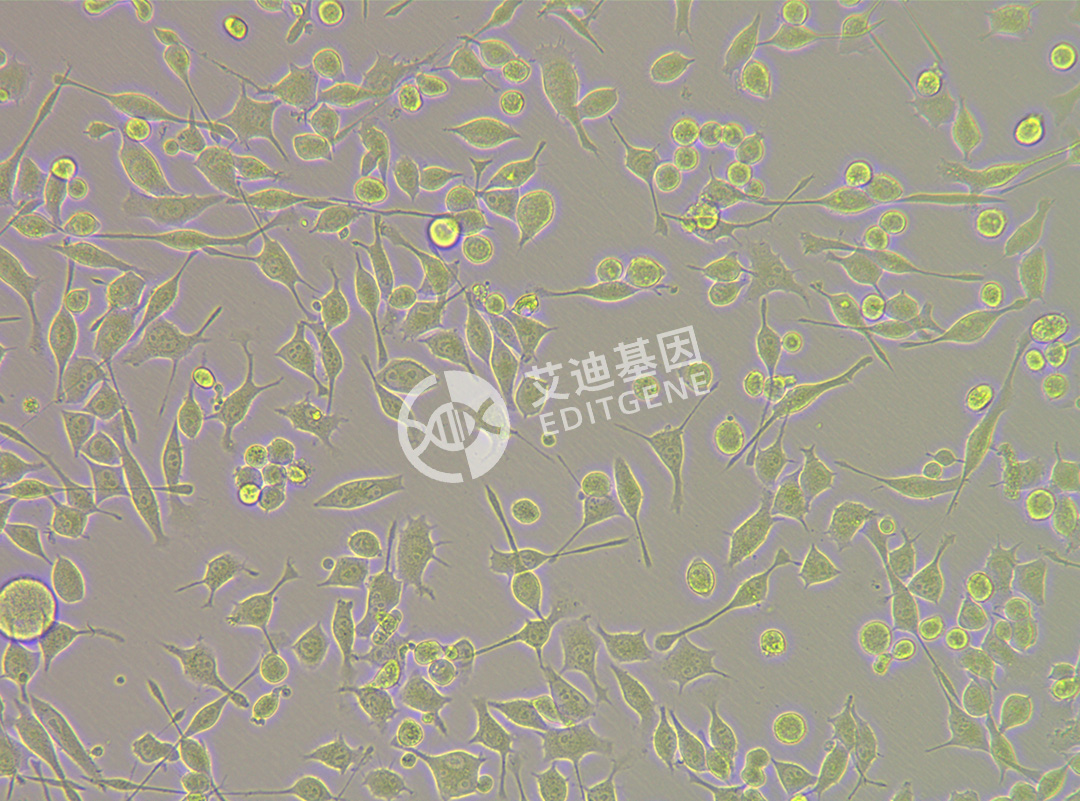
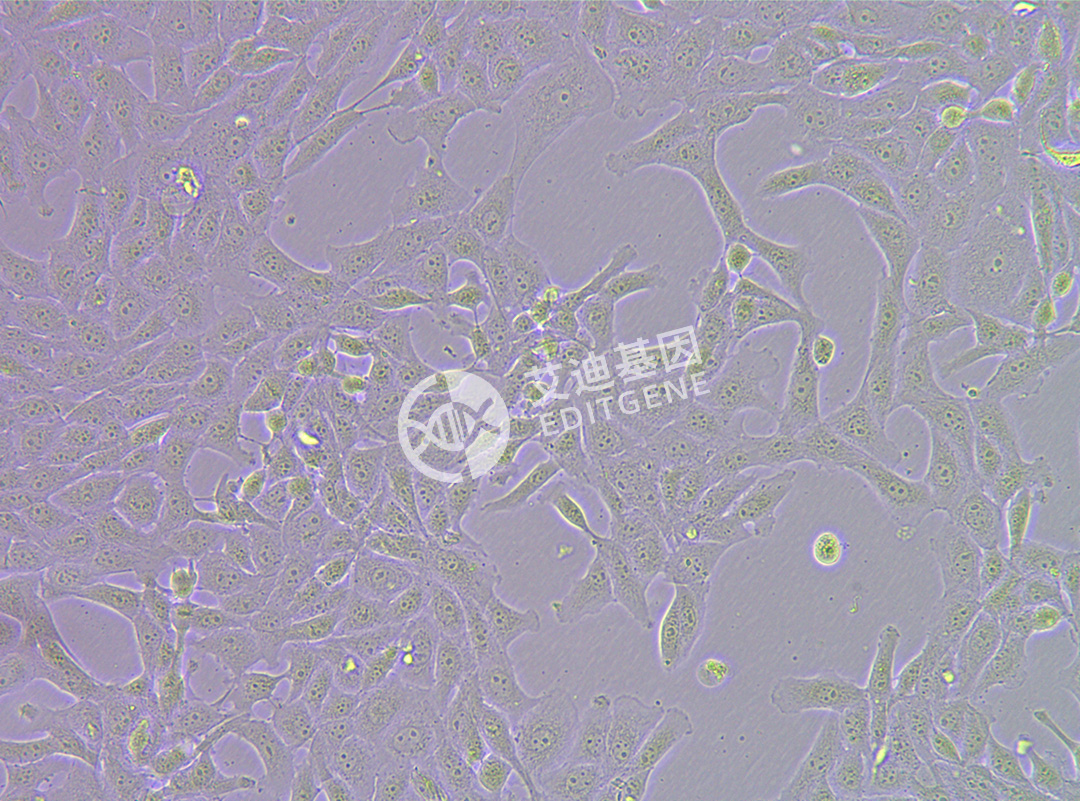
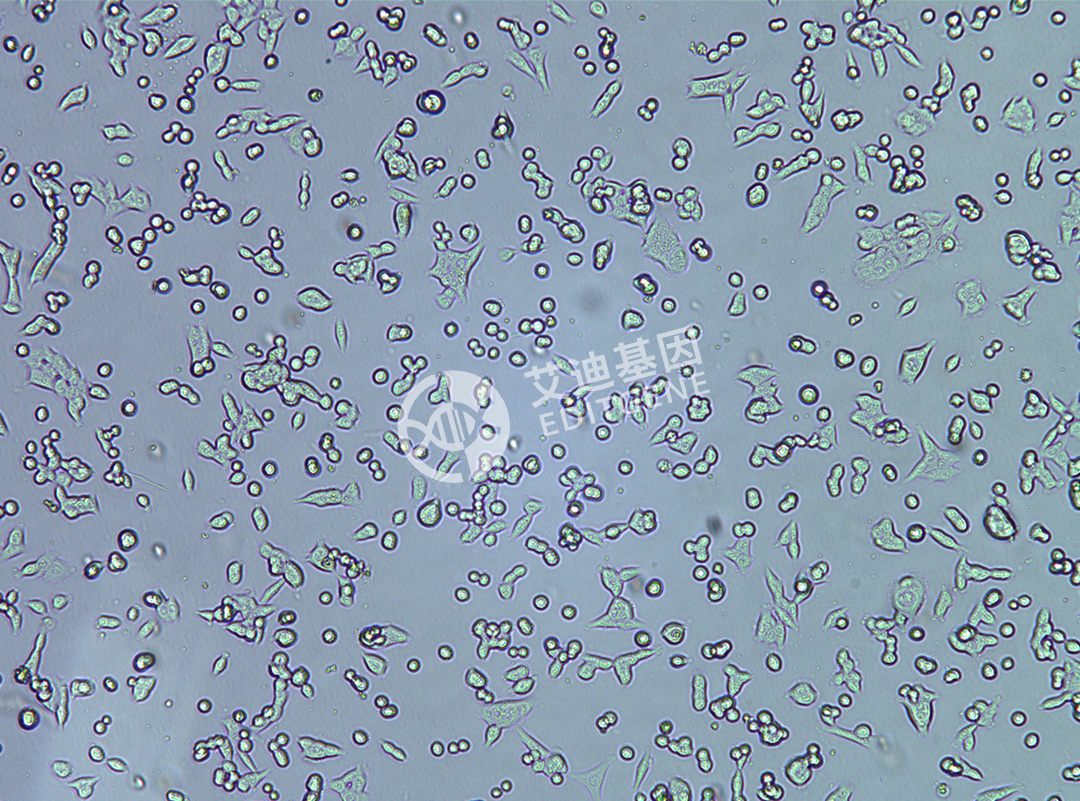
ISHIKAWA (Human endometrial cancer cells)
Cat.No.:
EDC2024053-W053
Passage Ratio:
1/3~1/4,2~3days
Introduction
Details Product Information
| Cat.No. | EDC2024053-W053 |
|---|---|
| Product Name | ISHIKAWA (Human endometrial cancer cells) |
| Morphology | Adherent |
| Passage Ratio | 1/3~1/4,2~3days |
| Complete Culture Medium | DMEM+10%FBS |
| Freezing Medium | 90%Complete culture medium+10%DMSO |
Cell recovery
Note: After receiving the cells, please store in liquid nitrogen. Take it out 10 minutes before recovering cells and place it at -80 ℃ to allow the liquid nitrogen in the tube to evaporate
1. Preheat the water bath and complete culture medium at 37 ℃;
2. Add 6mL of complete culture medium to a 15mL centrifuge tube;
3. Gently swirl the cryopreservation tube in a 37 ℃ water bath until a small piece of ice remains in the cryopreservation tube, please thaw it within 1 minute, and the cap should not touch water;
4. In the ultra clean bench, before opening the cap, wipe the outside of the cryopreservation tube with 75% alcohol;
5. Transfer all the liquid with a pipette to a centrifuge tube of preheated complete culture medium;
6. Rinse the cryopreservation tube with 1mL of complete culture medium to collect residual cells;
7. Centrifuge the cell suspension at 1100 rpm for 4 minutes (centrifugation speed and time depend on cell type);
8. After centrifugation, check if the supernatant is clear and if there is cell pellet at the bottom; inside the hood, carefully pour out the supernatant, add 1mL of complete culture medium, gently resuspend the cell pellet;
9. Evenly seed cells into a T25 flask or culture container with an equivalent bottom area. Add a sufficient amount of complete culture medium, and the total amount of culture medium in a T25 flask shall not be less than 6mL (the actual size of the flask depends on the number of cells frozen in the cryopreservation tube);
10. Gently mix the cells well and place them in a 37 ℃, 5% CO2, saturated humidity incubator (the culture environment depends on cell type and culture medium);
11. Observe the cell status on next day:
(1) If the adherent cells adhere well, change fresh complete culture medium; If the cells are observed to be in a round and bright shape but not adhering to the plate, continue cultivation for 24 hours. Afterwards, based on the growth status of the cells, replace the complete culture medium every 2-3 days, observe the cells, and passage cells when confluence rate >80%. If the cell growth is slow or the confluence rate is low, the frequency of medium change can be reduced;
(2) When recovering suspension cells, place them in a relatively small container and use a culture medium containing 20% serum for recovery. If the suspended cells are in good condition, change fresh complete culture medium; If the cell status is poor and appears grayscale, it can be further cultured for 72 hours. Change fresh medium if observe live cells. If no significant changes are observed, please contact us for after-sales service in a timely manner.
Passage method
1. Preheat the complete culture medium, PBS, and trypsin-EDTA to 37 ℃;
2. Vacuum and discard the supernatant;
3. Gently add PBS (~6mL for a T25 flask) to rinse the cells, then discard PBS, please avoid stirring the cell layer;
4. Add trypsin-EDTA (~3mL in a T25 flask) and quickly spread evenly to ensure full contact with the cell layer; Place it in the incubator for digestion;
5. Observe digestion under a microscope. After ~70%-80% of the cells become round, gently tap the flask to detach the cells;
6. Immediately add 2 times volume of complete culture medium (about 6mL in T25 flask), then gently shake to quickly mix the medium and trypsin to terminate digestion;
7. Use a pipette to gently blow the bottom of the culture container with the medium, and try to blow down all the cells as much as possible; Note: avoid to generate bubbles;
8. Transfer the cell suspension to a centrifuge tube. Wash the flask once with PBS (about 3mL added to T25 flask) and collect residual cells;
9. Centrifuge cell suspensions at 1100 rpm for 4 minutes;
10. Remove the supernatant after centrifugation. Add 1mL of complete culture medium, gently resuspend the cell pellet;
11. Seed cells in a certain passage ratio. It is recommended to pass the cells in a ratio of 1:3 for the first time. If the cells grow to >80% confluency within two days, the passage ratio can be increased. If the cells not grow to >80% confluency for three or four days, the passage ratio can be reduced;
Note: Please adjust the passage ratio based on the actual growth of the cells.
12. Place them in an incubator with 37 ℃, 5% CO2 and saturated humidity (if an culture flask is used, the cap should be loosened before putting them into the incubator to facilitate full Gas exchange, unless a ventilated flask or a breathable cap is used);
13. Observe the cell status on the next day. If there are a lot of dead cells, change to a fresh culture medium. Change fresh medium base on the cell growth. Cells need to be passaged when the confluency >80%.
Storage
Liquid nitrogen


 Login
Login

 download
download