[Literature Review] CRISPR Knock-In Strategy Enables Tumor-Restricted Payload Delivery
CRISPR knock-in

Chimeric antigen receptor (CAR) T-cell therapy has achieved remarkable success in treating hematologic malignancies. However, its effectiveness against solid tumors remains limited due to several challenges, including immune suppression within the tumor microenvironment, tumor antigen heterogeneity, and restricted CAR-T cell migration to tumor sites.
In July 2025, Nature published an online study titled "Rewiring endogenous genes in CAR-T cells for tumour-restricted payload delivery." The research team developed a CRISPR knock-in strategy that harnesses endogenous gene regulatory mechanisms to enable localized transgene expression within tumors. The results demonstrated significant antitumor efficacy without toxicity, improved survival across multiple mouse models, and applicability to patient-derived CAR-T cells.
Original link: https://doi.org/10.1038/s41586-025-09212-7
Spotlight
1. Superior Therapeutic Efficacy:
NR4A2/IL-12 mouse CAR-T cells demonstrated significantly enhanced antitumor activity across multiple tumor models. Even in advanced tumors larger than 30 mm², they effectively controlled tumor growth and markedly prolonged survival.
2. Increased Flexibility:
The CRISPR knock-in strategy allows selection of appropriate promoters tailored to the characteristics of the target transgene.
3. Simplified Clinical Manufacturing:
A “one-step” method enabled CAR targeting into the TRAC locus and knock-in of IL-12 or IL-2 into NR4A2 or RGS16 loci, respectively. CAR expression levels, knock-in efficiency, and in vivo efficacy matched those of the conventional “two-step” approach.
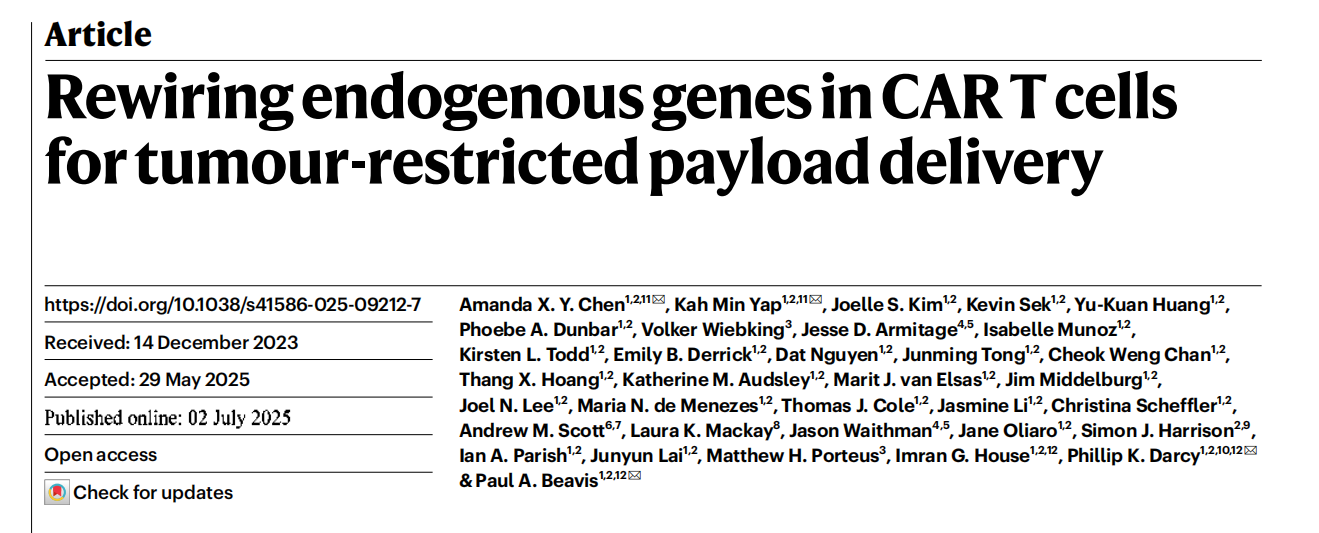
To validate the proposed CRISPR knock-in strategy, the researchers selected Pdcd1 as a prototype gene. They designed a homologous recombination repair template to insert green fluorescent protein (GFP) downstream of the Pdcd1 start codon and established an efficient editing protocol in primary mouse T cells.
Following stimulation, PD-1/GFP-edited OT-I cells exhibited a marked increase in GFP expression, demonstrating successful control of GFP expression via endogenous Pdcd1 promoter editing.
Upon adoptive transfer into mice, approximately 80% of OT-I cells within tumors expressed PD-1, whereas PD-1 expression was minimal in the spleen. These findings confirm that the Pdcd1 promoter can drive tumor-restricted transgene expression.
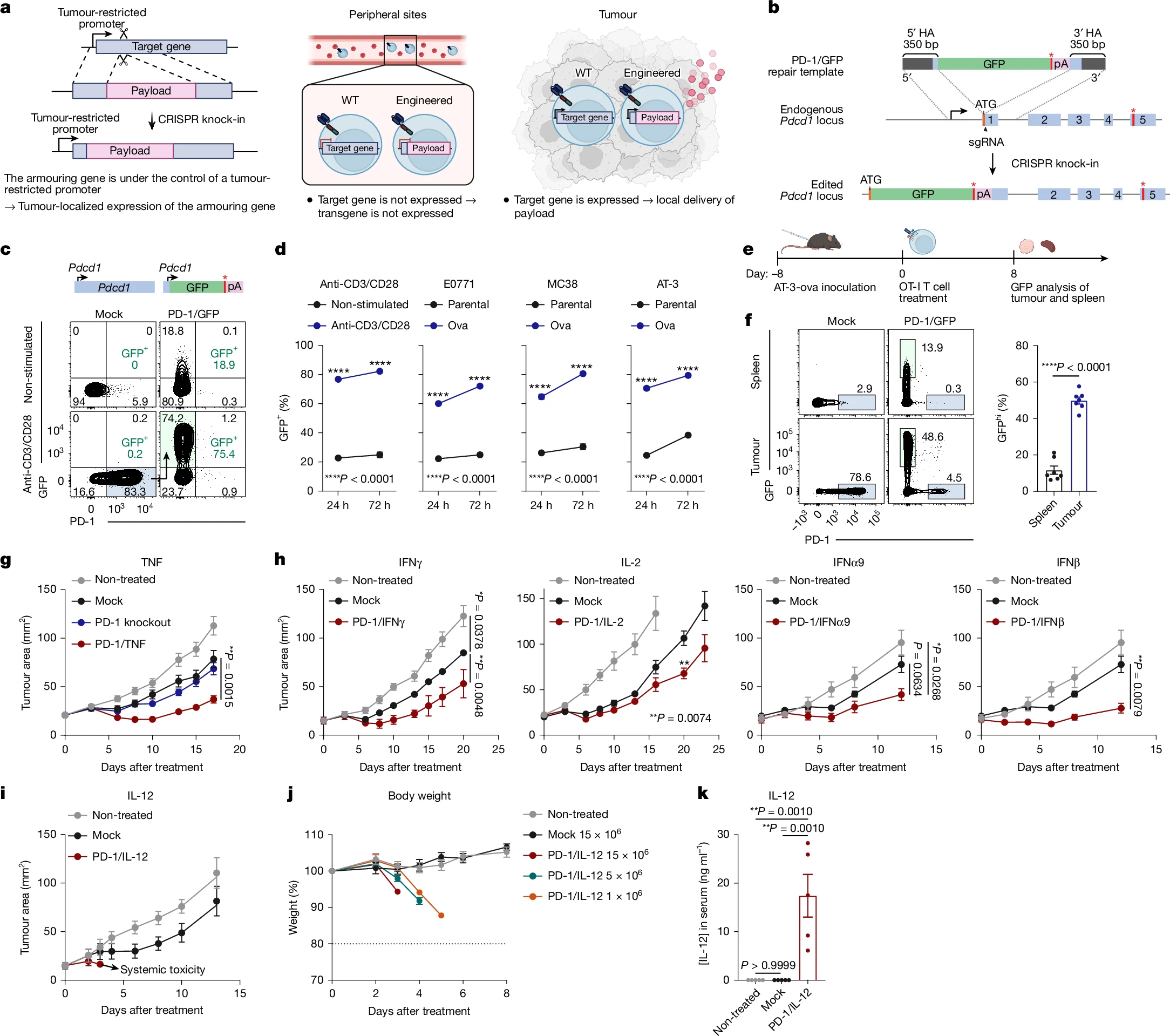
Figure 1. CRISPR knock-in strategy for developing armored T cells
To identify alternative target genes, the researchers performed RNA sequencing on CD8⁺ CAR-T cells isolated from cell models, preliminarily narrowing down 27 candidate genes based on dataset correlation.
Subsequently, gene knockouts of these 27 candidates were conducted in human anti-LeY CAR-T cells. Using cytokine production as the functional readout, the focus was further refined to genes including CLU, NR4A2, PDCD1, RGS1, RGS16, and RGS2.
Finally, by assessing the efficiency of tumor-specific GFP expression driven by these promoters, the researchers confirmed NR4A2 and RGS16 as the ideal promoters for targeted transgene expression.
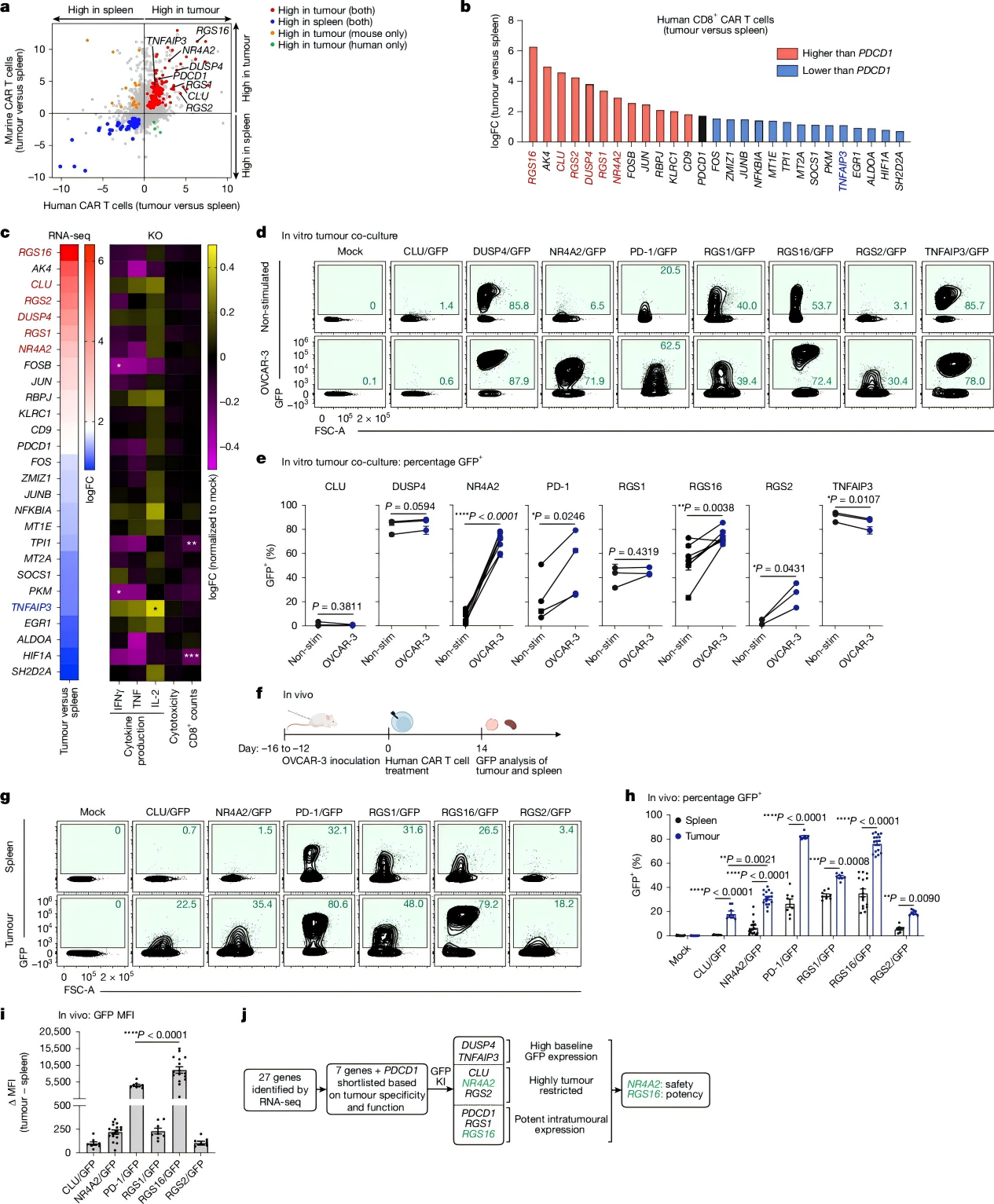
Figure 2. Identification of optimal target genes for CRISPR-engineered armored T cells
In efficacy and safety evaluations of NR4A2/IL-12 OT-I cells, significant antitumor effects were maintained even at low doses (1×10⁶ T cells). In contrast, the NR4A2 knockout group showed minimal therapeutic benefit, confirming that the observed efficacy primarily derives from localized IL-12 expression rather than NR4A2 deficiency.
In advanced large tumor models, NR4A2/IL-12 mouse CAR-T cells significantly improved survival without toxicity. Conversely, the NFAT-IL-12 group rapidly exhibited weight loss, hunching, and reduced activity.
These results demonstrate that the NR4A2 promoter restricts IL-12 expression specifically to tumor sites, thereby avoiding systemic toxicity.
Figure 3. Excellent Tolerability and Robust Antitumor Immune Response
To evaluate the strategy in human tumor models, the researchers first generated NR4A2/IL-12 human anti-LeY CAR-T cells, followed by the development of RGS16/IL-2 CAR-T cells.
The RGS16/IL-2 CAR-T cells not only achieved higher IL-2 expression levels but also significantly suppressed tumor growth in vivo without toxicity. Mechanistic studies revealed markedly increased expression of Ki67, IFNγ, and TNF in these CAR-T cells, along with significantly elevated numbers of CD8⁺ and CD4⁺ CAR-T cells in tumors, spleen, and peripheral blood.
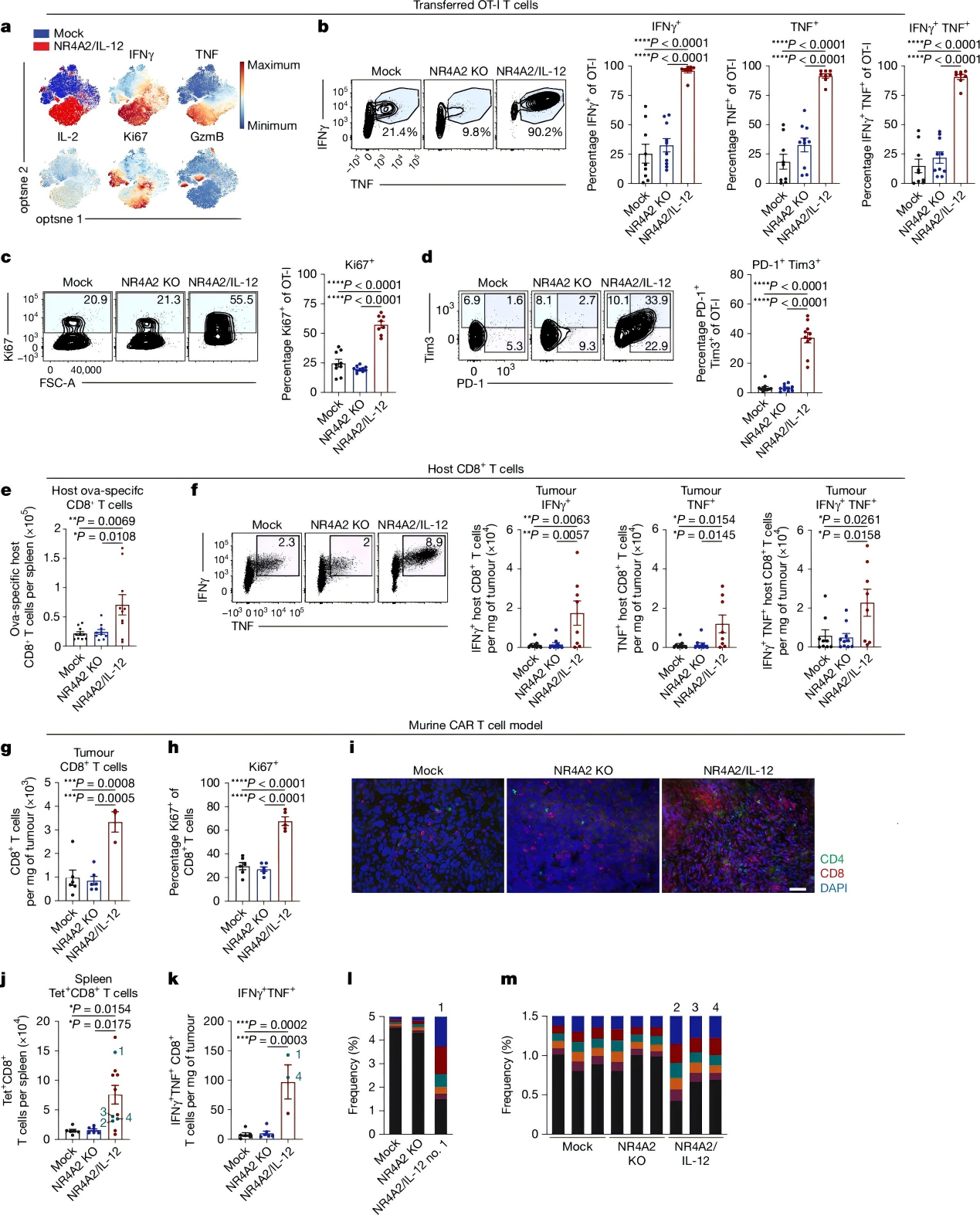
Figure 4. Enhanced proinflammatory response and promotion of host antitumor immunity
This study harnesses endogenous tumor-restricted promoters (NR4A2, RGS16) to drive precise expression of proinflammatory cytokines IL-12 or IL-2 within the tumor microenvironment. This approach significantly enhances CAR-T cell antitumor activity and extends long-term survival in mouse models, with demonstrated efficacy in patient-derived CAR-T cells as well.
Looking forward, this strategy holds promise to overcome current barriers in solid tumor therapy, offering a novel pathway to develop safer and more precise adoptive cell therapies.
















