[Literature Review] CCR5 Knockout Combined with Antibody Expression Powers a Multi-Layered Defense Against HIV
gene knockout

Despite decades of research, HIV infection remains incurable. Current antiretroviral therapies require lifelong adherence and are ineffective at eliminating latent viral reservoirs. However, the cases of the "Berlin patient" and the "London patient" — both functionally cured — have drawn significant attention to hematopoietic stem cell transplantation (HSCT) using CCR5-deficient donor cells. These landmark cases have sparked widespread scientific interest in CCR5 gene knockout as a potential therapeutic target for HIV.
In a 2025 publication in Nature Communications titled “Multilayered HIV-1 resistance in HSPCs through CCR5 knockout and B cell secretion of HIV-inhibiting antibodies,” Matthew Porteus team from Stanford University proposed an autologous hematopoietic stem cell gene editing approach. By integrating CCR5 knockout with engineered antibody secretion, the team established a multi-layered HIV defense system, offering a promising step toward functional cure.

Original link: DOI: 10.1038/s41467-025-58371-8
Spotlight
1. CRISPR-Cas9 gene editing was applied to autologous hematopoietic stem cells (HSPCs), endowing them with both intracellular and extracellular mechanisms of resistance against HIV.
2. In addition to knocking out the CCR5 gene, the researchers precisely inserted a broadly neutralizing antibody (bNAb) gene, enabling the edited HSPCs to differentiate into B cells that continuously secrete potent anti-HIV antibodies.
3. This dual-action strategy offers the potential for a one-time functional cure: following a single transplantation of gene-edited autologous HSPCs, patients may achieve long-term viral control through sustained production of HIV-resistant immune cells and neutralizing antibodies—eliminating the need for lifelong antiretroviral therapy.
The researchers utilized high-fidelity Cas9 protein complexed with sgRNA targeting CCR5 to form a ribonucleoprotein (RNP) complex, which was electroporated into hematopoietic stem and progenitor cells (HSPCs). Simultaneously, an AAV6 vector was used to deliver the donor DNA template. Through homology-directed repair (HDR), the researchers achieved precise CCR5 gene knockout along with site-specific knock-in of antibody genes. The inserted genes encoded broadly neutralizing antibodies (bNAbs) Ibalizumab and 10-1074, providing coverage against multiple HIV-1 subtypes.
Remarkably, this strategy achieved a 50% knock-in efficiency and a CCR5 knockout rate exceeding 90%, with robust multilineage engraftment and sustained antibody expression observed in animal models.
Furthermore, the study incorporated the DNA-PK inhibitor AZD7648, which significantly increased biallelic knock-in rates from 21% to 51% without detectable toxicity or impairment of cellular differentiation—further underscoring the clinical potential of this approach.
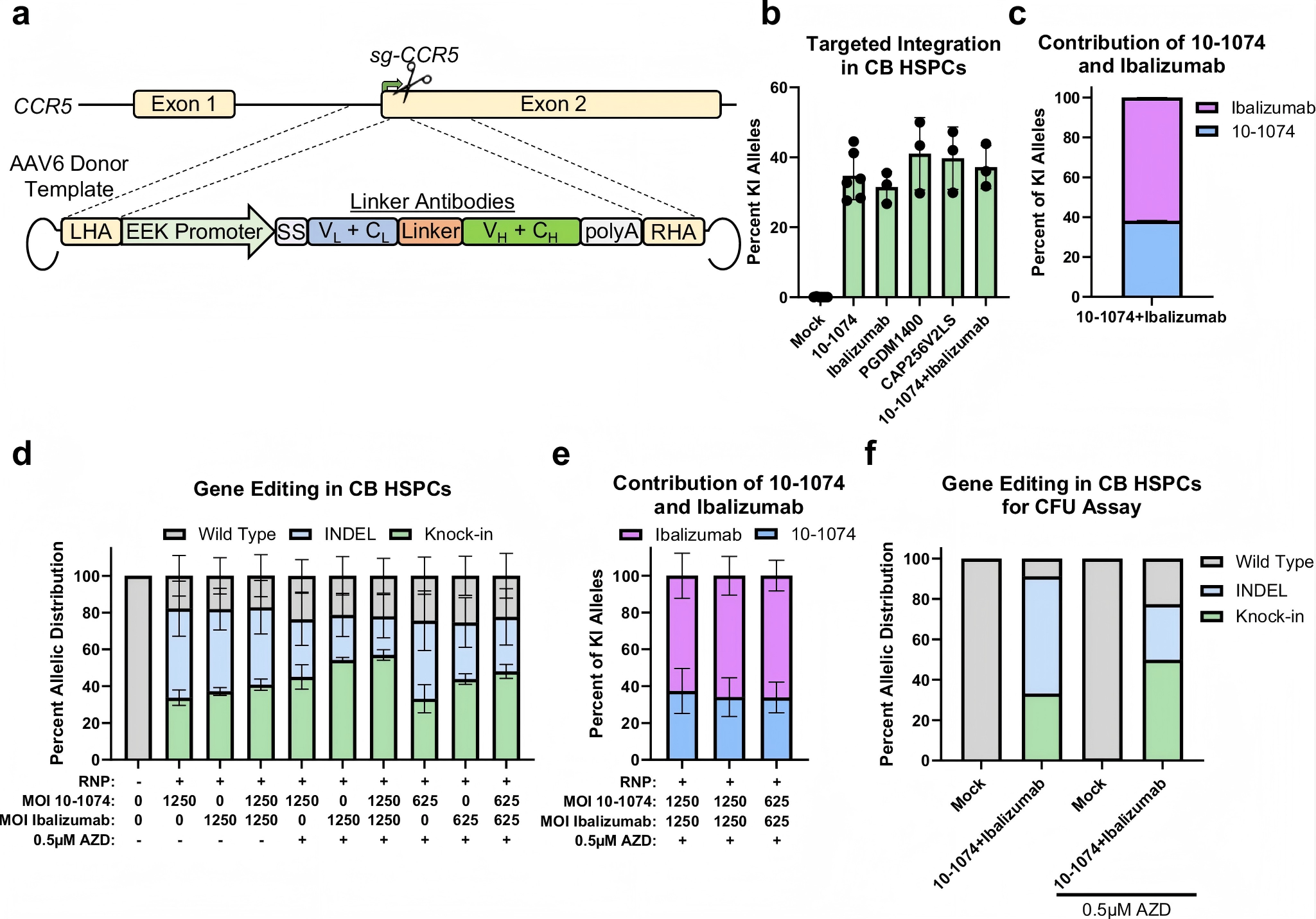
Figure 1. Efficient targeted integration of antibody expression cassette into the CCR5 locus of hematopoietic stem cells
The edited HSPCs were transplanted into NSG immunodeficient mice, and after 16 weeks, human cell chimerism was successfully detected in both the bone marrow and spleen, along with stable and sustained expression of anti-HIV antibodies.
Given the limited ability of human B cells to fully differentiate in murine models, the researchers further conducted in vitro editing experiments on peripheral blood-derived B cells, confirming that cells carrying the antibody expression cassette were capable of efficiently secreting broadly neutralizing antibodies (bNAbs) and demonstrated effective viral suppression. These findings further validate the efficacy and therapeutic potential of the proposed strategy.
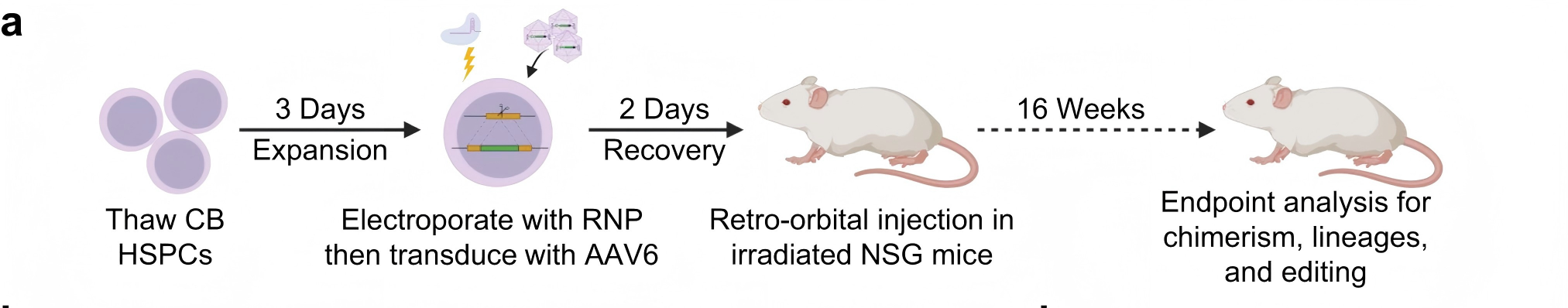
Figure 2. Long-term persistence and antibody expression of edited HSPCs in mice
Compared to traditional CCR5 knockout strategies alone, this multi-layered defense system significantly enhances broad-spectrum resistance to HIV-1, effectively targeting both R5- and X4-tropic strains, and reducing the risk of viral escape mutations.
The study demonstrates that engineered HSPCs can maintain normal immune function while sustainably producing HIV-neutralizing antibodies over the long term. This strategy holds promise to replace the current “patchwork” treatment model—which relies on frequent injections—with a one-time, functionally curative approach.
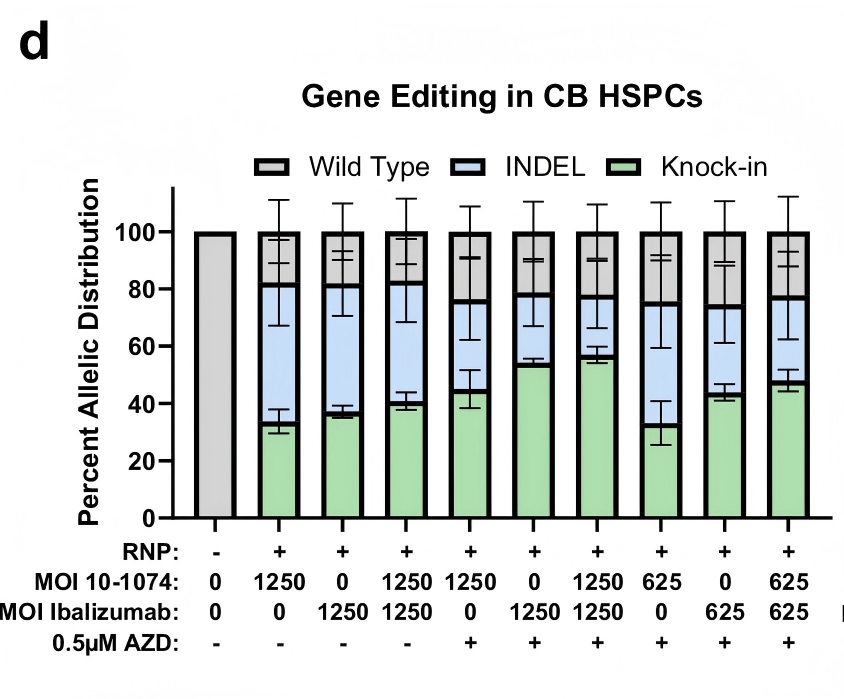
Figure 3. Inhibition of viral entry by edited cells under infection with different HIV subtypes
This study demonstrates the use of CRISPR-Cas9 gene editing technology for precise gene knockout, coupled with diversified antibody gene design, enabling multiplexed antibody editing strategies and offering novel insights into personalized HIV therapy. By allowing flexible combinations of neutralizing antibodies tailored to different HIV subtypes, this approach effectively suppresses viral escape and minimizes the risk of drug resistance.
More promisingly, this modular platform is not limited to HIV. With continued advancements in gene editing, the system holds great potential for expansion into antibody-based therapies for other chronic diseases, including hepatitis B and cancer, paving the way for a broader future of individualized treatment.
















