[Literature Review] Cellular “Logic Gate” Technology: RNA Switches Pave the Way for Precision Therapies
CRISPR-Cas9
RNA

In July 2025, Nature Communications published the article “Split RNA switch orchestrates pre- and post-translational control to enable cell type-specific gene expression”, presenting a novel gene regulation technology called the “Split RNA Switch.” This system integrates output signals from multiple RNA switches and employs a protein splicing mechanism to achieve precise control of target gene expression.
Original link: https://doi.org/10.1038/s41467-025-60392-2
Spotlight
1. 25-45× Increase in ON/OFF Ratio
The combination of the Split RNA switch with a leak-expression suppressor reduced background noise to 1/25-1/45 of the original level, achieving, for the first time, antibiotic-based selection and purification using only synthetic mRNA.
2. One mRNA “Puzzle” Compatible with Multiple Functions
Fluorescent proteins, antibiotic resistance genes, Cas9, and other effectors can be split into two segments and reconstituted, demonstrating that this mRNA platform is plug-and-play and compatible with virtually any downstream effector protein.
3. Programmable Cellular Logic Circuits
By employing two sets of orthogonal split inteins, the researchers constructed a three-input logic gate capable of simultaneously recognizing three distinct miRNAs, providing a new tool for precise classification of complex cellular subpopulations.
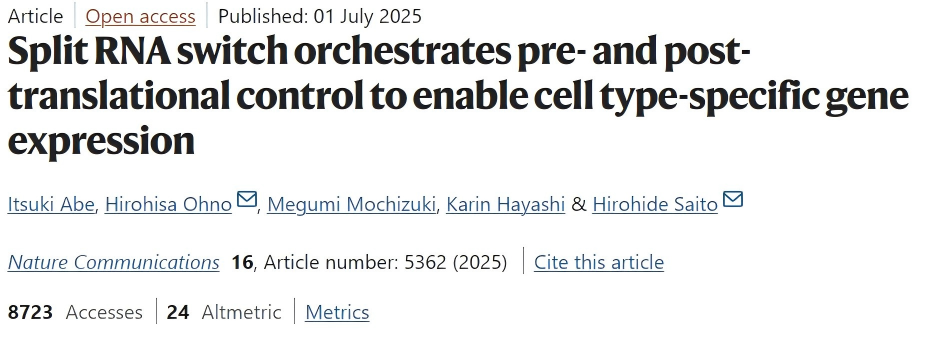
First, the target protein, such as CRISPR-Cas9, is split into N-terminal and C-terminal fragments, each fused to a split intein. When both fragments are present in the cell, the split intein catalyzes their ligation to reconstitute a fully functional protein.
Two types of RNA switches—ON and OFF switches—are then designed. The ON switch promotes target protein expression in the presence of the target miRNA, whereas the OFF switch suppresses target protein expression under the same conditions.
The N- and C-terminal protein fragments are encoded by two separate ON switches, and an OFF switch encoding a mutated C-terminal fragment is introduced as a “leak-expression suppressor.” In miRNA-negative cells, any low-level leak expression of the N-terminal fragment from the ON switch is neutralized by the mutated C-terminal fragment expressed from the OFF switch, forming a nonfunctional complex and effectively suppressing background noise.
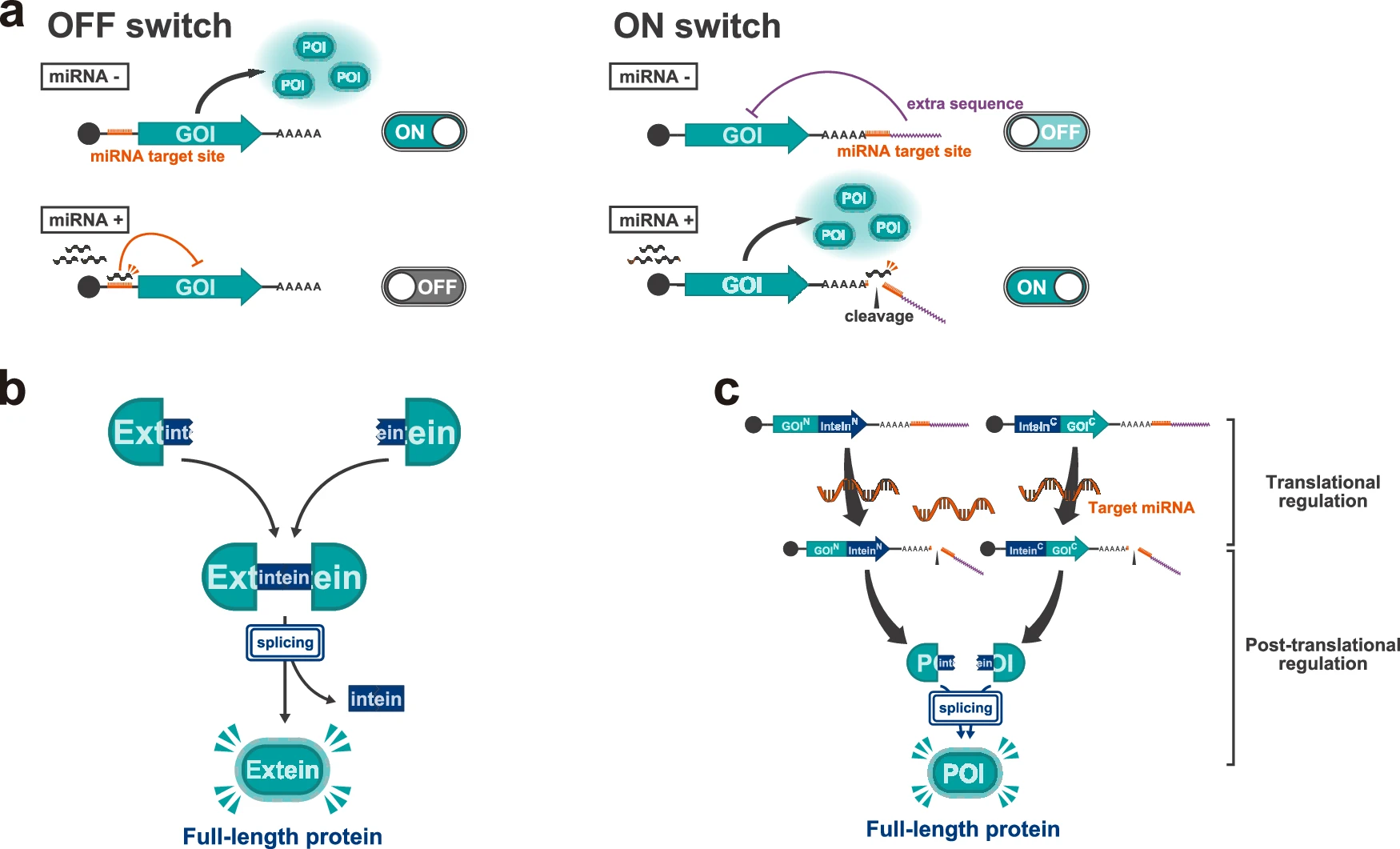
Figure 1. Schematic of ON and OFF switches
1. The researchers first tested the performance of the Split RNA Switch in HEK293FT cells. Using miR-21-5p as the target miRNA, they quantitatively analyzed the expression of the fluorescent protein hmAG1 via flow cytometry and fluorescence microscopy.
The results showed that the Split RNA Switch system markedly enhanced fluorescent protein expression in miRNA-positive cells, while suppressing background noise in miRNA-negative cells, increasing the ON/OFF ratio from 3-fold with a single switch to over 25-fold.
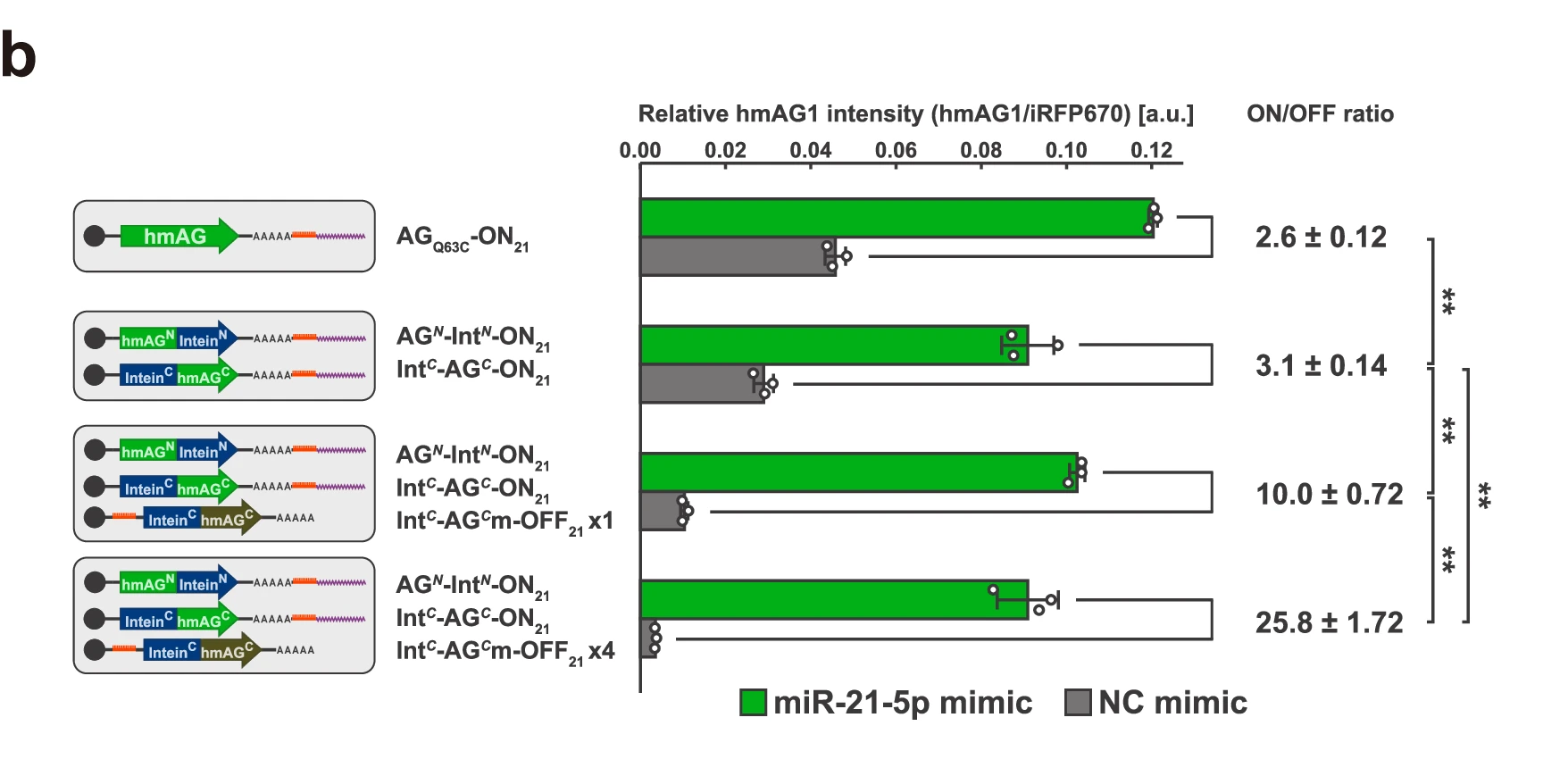
Figure 2. Addition of leak-expression suppressor increases ON/OFF ratio to over 25-fold
2. The researchers further evaluated the application of the Split RNA Switch in controlling the expression of antibiotic resistance genes such as PAC, HPH, and BSR.
By introducing the Split RNA Switch system into HeLa cells and culturing under different conditions, they found that the system effectively distinguished miRNA-positive from miRNA-negative cells, achieving cell type-specific antibiotic resistance. In mixed HeLa/HEK293FT populations, the purity of target cells was increased from 58% to 96%.
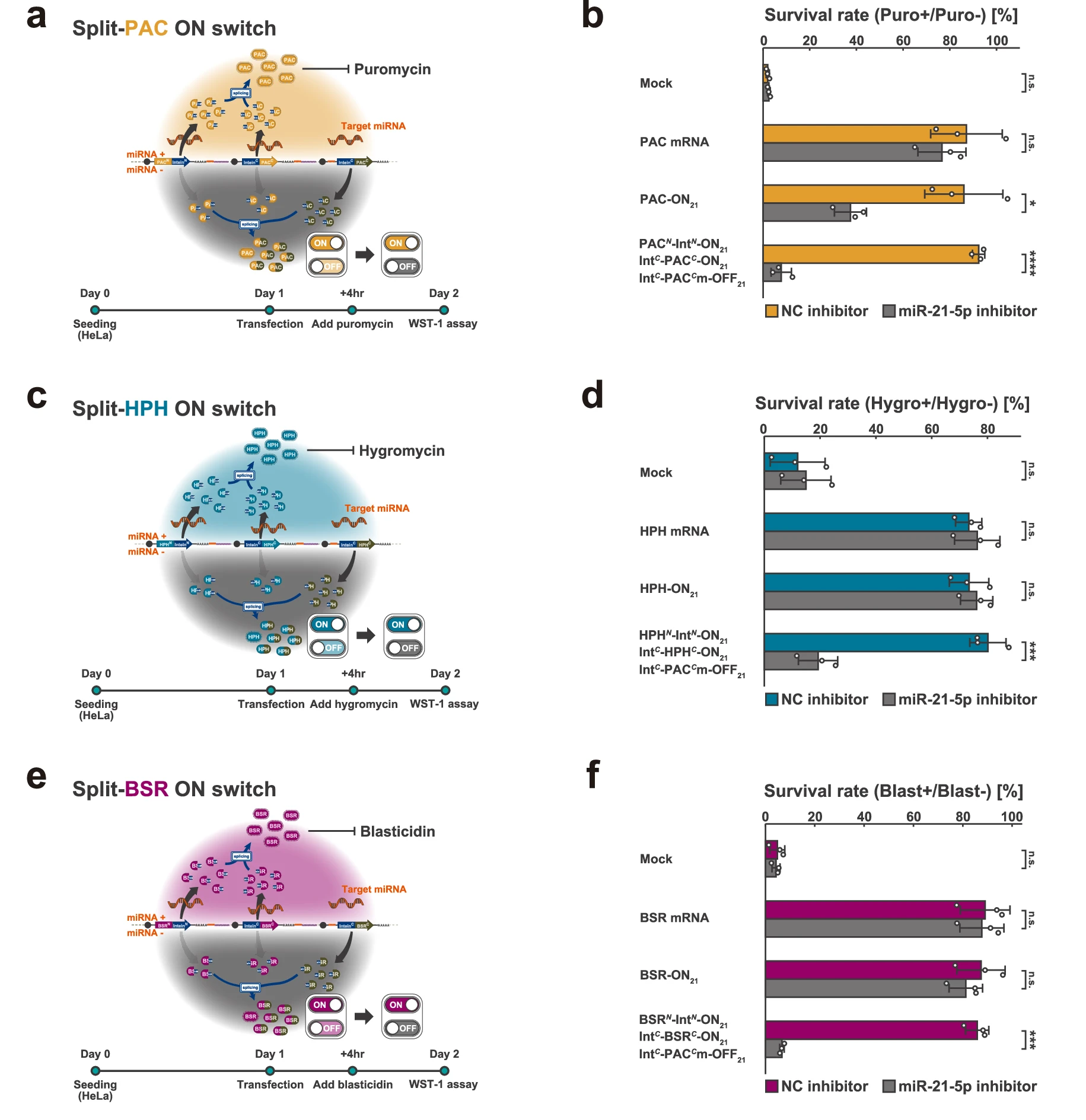
Figure 3. Split RNA-controlled antibiotic resistance genes enable >80% cell survival in HeLa cells
The researchers used the Split RNA Switch system to construct various two-input logic gates. By combining different miRNA-responsive switches, they were able to simultaneously detect multiple miRNA signals and perform logical operations.
Additionally, by employing two sets of orthogonal split inteins, they successfully built a three-input logic gate capable of detecting and integrating three distinct miRNA signals concurrently.
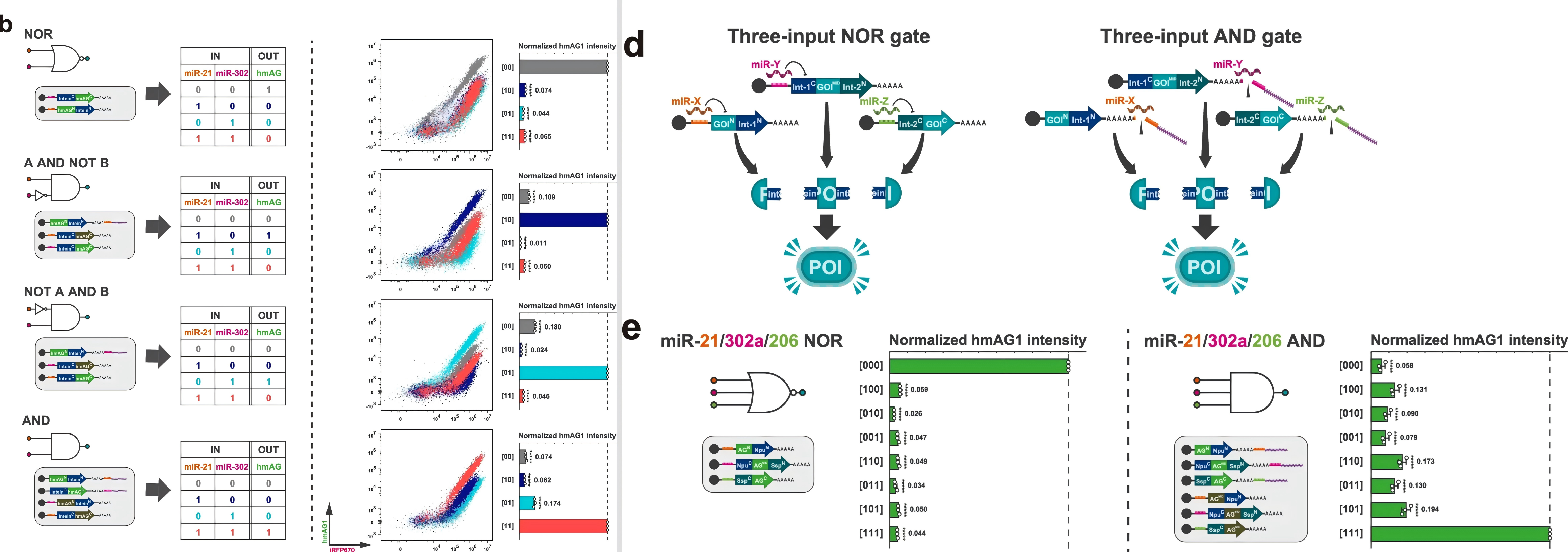
Figure 4. Constructed two-input (left) and three-input (right) biological logic gate circuit structures
The Split RNA Switch system employs a three-step “cut-splice-neutralize” strategy to simultaneously address leak expression and marker deficiency. Using only mRNA delivery, it achieves highly specific cell purification, gene editing , and safe suicide functions, compatible with existing mRNA platforms such as liposomes and LNPs.
Looking ahead, logic gate versions can be expanded to accommodate any number of inputs, potentially functioning like a “cellular GPS” to guide therapies within complex tissues. However, considerations remain: the leak-expression suppressor may slightly reduce ON-state activity, RNA delivery efficiency must be optimized for in vivo applications, and logic gate response speed should be improved to facilitate clinical translation.
















